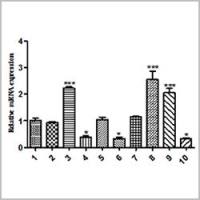
Identification of Homozygous Transgenic Mice by Genomic Real-Time PCR
互联网
654
The 26S proteasome is the executing protease of the ubiquitin-dependent degradation system. It consists of one or two 19S regulatory sub-complexes and one 20S proteolytic sub-complex (1 ). The 20S proteasome is a barrel-shaped cylinder which consists or four stacked rings (2 ). Each of the two outer rings consists of seven different α-subunits, whereas each of the two inner rings is formed by seven different β-subunits (3 ). Only three of these β-subunits bear a catalytically active N-terminal threonine (4 ,5 ). Under normal conditions, these are β1 (delta), β2 (Z), and β5 (mb1). However, by induction of some cytokines, e.g., interferon-γ, these subunits are exchanged against β1i(LMP2), β2i (Mecl1), and β5i (LMP7) and the so-called immunoproteasome is formed (6 ,7 ). To investigate the role of LMP7 in MHC class I-restricted immunology, we decided to generate a transgenic mouse which constitutively expresses LMP7 in all tissues. To get the highest possible expression, we bread the mice to be homozygous for the transgene LMP7. These mice cannot be identified by conventional polymerase chain reaction (PCR). So far, Southern blotting was the only possible method to quantify the DNA content. Here, we describe the analysis of these mice by quantitative PCR using sequence specific fluorescence resonance energy transfer-primers to reliably detect a difference in DNA content as small as a factor of 2 or only one PCR cycle.