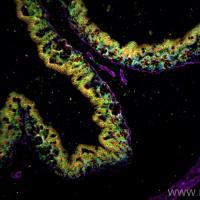
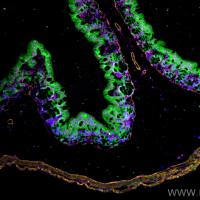
BrdU Labeling Protocol
互联网
Materials Required But Not Provided
1. BrdU reagent (Invitrogen Cat. No. 00-0103)
Note: For larger animals use 10 ml per 1 kg of body weight.
2) Wait 2 hours and sacrifice the animal. Remove the organs you wish to study.
3) Process the tissue as necessary (frozen sections or paraffin embedding).
4) Cut sections for immunohistochemistry.
2. Labeling Culture Cells or Cell Suspensions in Flask
3. Labeling Culture Cells on Chamber Slides
1) Culture cells overnight on 4-well chamber slides.
3) Remove medium from chamber slides and replace with labeling solution.
5) Remove labeling medium from cells and wash gently with PBS, 2 times.
6) Fix cells with 70-80% alcohol or acid alcohol for 20-30 min.
7) Wash with PBS (3 times, 2 min. each)
8) Proceed with immunocytochemical staining techniques.
4) Wash tissue slices in PBS at 37°C (3 times, 5 minutes each).
5) Fix the tissue using a frozen section protocol or embed in paraffin as necessary.