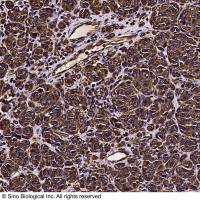
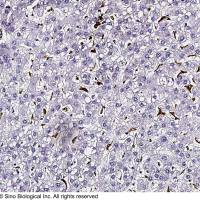
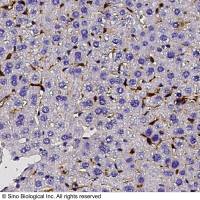
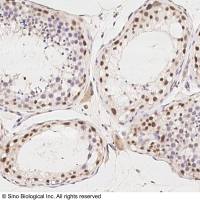
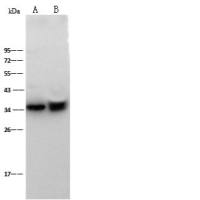
Integration of Extraction with Affinity Precipitation
互联网
337
Conventional aqueous two-phase extraction by spontaneous partitioning has often problems with low specificity. The introduction of an affinity ligand into one of the phases has been attempted to enhance the specificity of protein partitioning (1-7; Chapters 29-31). However, this procedure still has some limitations in the effective removal of impurities as well as recovery and reuse of the ligand. During the past decade, another potentially scalable technique, affinity precipitation, has been studied for the isolation of proteins (8 -10 ). The affinity ligand coupled to a reversibly soluble-insoluble polymer is used to specifically bind the protein in a complex mixture. The precipitation step which can be accomplished by a change in any environmental parameter such as pH, temperature, salinity, and so forth, facilitates the separation of the bound ligand and affinity complex from the unbound components. Although cells and cell debris should be removed before use, recovery and reuse of the ligand are relatively easy.