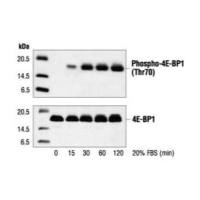
植物叶蛋白western blotting
互联网
3487
1. Weigh ~0.5g fresh leaf tissue (3-5cm2 ), grind using liquid nitrogen.
2. Add ~0.4ml Plant Extraction Buffer, grind until slurry, transfer to 1.5ml tube.
3. Spin down for 15 minutes at 4°C, transfer 0.2-0.3 ml supernatant to fresh tube.
4. Add an equal volume of 2X Laemmli's buffer, boil for 5 min.
5. Use immediately to load gel or store at -20°C (reboil before loading gel).
II. Pour Acrylamide Gel:
|
Running gel, lower (10%)(~30 mls for 2 gels:
Acrylamide (40%)dH2 O 1M Tris (pH 8.7) 10% SDS 10% APS (Ammonium persulfate) TEMED |
11.0ml 11.2ml 300µL 120µL 20µL |
Mix and pour gel, then layer on top with butanol; allow to polyermize (30 min.), rinse off the butanol with H2 O before layering on Stacking Gel.
|
Stacking gel, upper (for 2 gels): Acrylamide (40%)
dH2 O 1M Tris-HCl (pH 6.8) 10% SDS 10% APS TEMED |
7.25ml 1.3ml 104µl 52µl 11µl |
Insert comb, allow to polymerize (30 minutes); remove comb just before loading samples.
III. Gel Electrophoresis:
1. Setup gel in electrophoresis apparatus, fill reservoirs with 1X Running Buffer, check to remove air bubble from between plates at bottom of gel.
2. Remove comb and rinse wells with 1X Running Buffer.
3. Boil samples and standards for 5 min., then load gel (15-25µl for Hoefer mini-gel, 20-50µl for midi-gel).
4. Run until BPB-dye reaches bottom of gel (about 1-1.5hr for mini-gel at 40mA, about 2-2.5 hours for midi-gel at 200V).
5. If staining protein, cut away stacking gel and place running gel in Coomassie Blue Stain.
6. Destaining:
Decant off stain and soak gel in Destain I
Transfer gel into Destain II until background removed
Observe on light box
Vacuum dry for permanent record and/or densitometric analysis.
IV. Gel to Filter Blotting:
1. Remove gel from plate-sandwich (note L to R orientation), cut away stacking gel and agar plug (if use), rinse running gel briefly in semi-dryTransfer Buffer (sdTB):
2. Cut six Whatman filter papers and one Immobilon-P filter to the size of the gel, soak Immobilon-P filter in methanol for 5 seconds, and then wet all filters with sdTB.
3. a) Lay 2-gel plastic manifold in Hoefer Electro-blotter.
&nsbp; b) Lay 2 wetted filter papers for each gel onto platform of Blotter.
&nsbp; c) Place Immobilon-P filter onto filters (need to mark or cut notch on desired corner of filter to maintain gel-lane orientations).
d) Place gel in known orientation onto Immunobilon-P filter.
e) Place 3 more wetted filters on top of stack, removing bubbles by rolling pipet.
f) Install top of Electro-blotter and run at 150mA for 30-40 minutes (maximum 1 hour).
4. Remove Immunobilon filter(s), rinse with TBSt, and mark MW bands andgel top (T) with lab marker or pencil.
5. Can begin western probing immediately or store filter, either
a) rinse in TBSt, place in seal-bag and store at 4°C, or
b) place in Blocking Solution in seal-bag and store at -20°C.
Note: Be aware of what type of membrane you are using as it could affect how you want to store.
V. Probing the Filter:
1. Rinse filter in TBSt for ~5 min, then place in seal-bag with 10-20ml Blocking Solution for at least one hour at room temperature:
Blocking Solution:
10-20% goat serum albumin
5% Carnation non-fat dry milk
in TBSt buffer
2. Add Primary Antibody (1µg/ml) directly to Blocking Solution in seal-bag, incubate at room temperature for 1-2 hours.
3. Transfer filter to small dish and wash 3X 5 minutes with TBSt.
4. Place filter in seal-bag with 10-20 ml Blocking Solution and add Secondary Antibody to the dilution of 1:2000 (probably AP-labelled, sheep anti-mouse IgG), incubate at room temperature for ~30 minutes.
5. Wash filters with two quick rinses, then 3X 5 minutes with TBSt.
6. To develop with ECL Chemifluorescent Kit: -mix equal parts of ECL solutions A and b (~1 ml each per filter)
-spread ECL Solution Mix onto protein side of filter and incubate for 1 minute
-drain filter and wrap in plastic wrap, carry to darkroom in film cassette, and expose to ECL Hyperfilm. Exposure times range from 30 sec to 10 min.
Western Blot Reagents:
|
Plant Extaction Buffer (final concentration):
10mM NaCl10mM MgCl2 5mM EDTA 10mM β-mercaptoethanol 25mM Tris-HCl (pH 7.5) 1mM PMSF |
|
Laemmli's Sample Buffer (2X):
1.0M Tris-HCl (pH 6.8)10% SDS glycerol (37.7g) β-mercaptoethanol bromophenol blue -raise to 100ml with dH2 O |
12.5ml 40ml ~30ml 1ml "a pinch" |
|
Laemmli's Running Buffer (10X, dilute to 1X for PAGE):
-start with approx. 600ml dH2 O, add:Trizma Base glycine 10% SDS -raise to 1L with dH2 O |
30.28g 144.14g 100ml |
|
Coomassie Blue Stain (for 500ml):
glacial acetic acidmethanol -raise to 500ml with dH2 O, add 2g Coomassie blue G-250 |
100 ml 250ml |
|
Destain I (for 1L):
methanolglacial acetic acid -raise to 1L with dH2 O |
100ml |
|
Destain II (for 1L):
methanolglacial acetic acid -raise to 1L with dH2 O |
70ml |
|
TBS (for 1L):
1.0M Tris-HCl, pH 7.55.0M NaCl -raise to 1L with dH2 O, adjust pH to 7.5 |
30ml |
|
TBSt (for 4L):
-start with approx. 3L dH2 O, add:Trizma Base NaCl -raise to 4L with dH2 O, adjust pH to 7.5, then add ~4ml Tween 20 |
9.6g 32g |
|
semi-dry Transfer Buffer (sdTB, for 400ml):
-start with approx. 250ml dH2 O, add: Trizma Base glycine methanol -raise to 400ml with dH2 O |
1.2g 5.66g 80ml |
|
Blocking Solution (make fresh, for 50ml):
-into clean, 50ml conical tube, weigh: Carnation non-fat, dry milk -dissolve into approx. 30ml TBSt, add: Goat Serum Albumin -raise to 50ml with TBSt |
2.5g (5%) 5-10ml (10-20%) |
上一篇:Progeny analysis of plants 下一篇:消毒种子--Sterilizing Seed