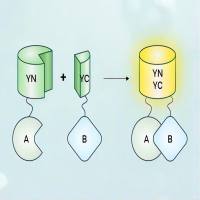
Cells co-express multiple G protein β and γ subunit isoforms, but the extent to which individual subunits associate to form particular βγ complexes is not known. This issue is important because in vivo knockout experiments suggest that specific βγ complexes may have unique functions despite the fact that most complexes exhibit similar properties when assayed in reconstituted systems. This chapter describes how multicolor bimolecular fluorescence complementation (BiFC) can be used in living cells to study the association preferences of β and γ subunits. Multicolor BiFC determines the association preferences of these subunits by quantifying the two fluorescent complexes formed when β or γ subunits fused to amino terminal fragments of yellow fluorescent protein (YFP-N) and cyan fluorescent protein (CFP-N) compete for interaction with limiting amounts of a common γ or β subunit, respectively, fused to a carboxyl terminal fragment of CFP (CFP-C). One means by which βγ complexes may differ from each other and thereby mediate unique functions in vivo is in the kinetics and patterns of their internalization responses to stimulation of G protein-coupled receptors (GPCRs). Methods are described for imaging and quantifying the internalization of pairs of βγ complexes in response to GPCR stimulation in living cells.