相关产品推荐更多 >
万千商家帮你免费找货
0 人在求购买到急需产品
- 详细信息
- 询价记录
- 文献和实验
- 技术资料
- 库存:
999
- 英文名:
SARS-CoV-2 (2019-nCoV) Nucleocapsid Protein (His tag)
- 保质期:
12个月
- 供应商:
北京义翘神州科技股份有限公司
- 保存条件:
-20℃ to -80℃
- 规格:
100 µg
新冠病毒N蛋白是义翘神州自主研发生产的SARS-COV-2核衣壳重组蛋白,具有高纯度、高活性、低内毒素的特点,可以用于新冠病毒的抗体筛选、药物研发、疫苗研发、致病机理等方面的研究。
新冠病毒N蛋白具有如下的优势:
1. 新冠病毒N蛋白经过SDS-PAGE电泳检测,蛋白纯度> 90 %.
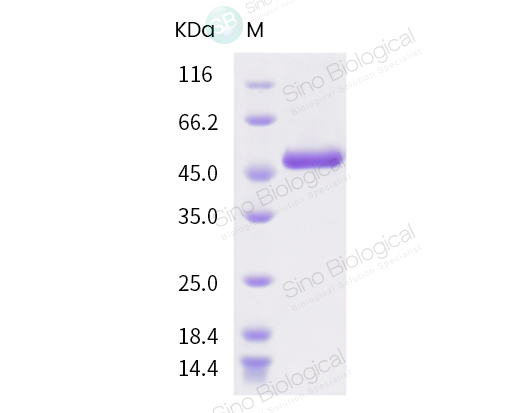
2. 新冠病毒N蛋白内毒素利用LAL方法检测<1.0 EU/μg。
3. 新冠病毒N蛋白是由SARS-COV-2 Nucleocapsid Protein基因表达,并且在C-段含有一个多组氨酸标签。
4. 新冠病毒N蛋白由义翘神州构建的杆状病毒-昆虫细胞表达系统制备,生物活性高。
5. 新冠病毒N蛋白的生物学活性正在检测中。

点击图片,了解更多促销信息
北京义翘神州科技股份有限公司(Sino Biological Inc.)是一家从事生物试剂研发、生产、销售并提供技术服务的生物科技公司,主要业务包括重组蛋白、抗体、基因和培养基等产品,以及重组蛋白、抗体的开发和生物分析检测等服务。
义翘神州为全球的药品研发企业和生命科学研究机构提供高质量的生物试剂产品和高水平的技术服务,能够覆盖生命科学研究的多个领域,为分子生物学、细胞生物学、免疫学、发育生物学、干细胞研究等基础科研方向和创新药物研发提供“一站式”生物试剂产品和技术服务。客户涵盖大学、科研院所、医药研发企业等国内外各类生物研发单位。目前公司已经在美国、欧洲等地建立了子公司,在上海建立分公司,已成为生物试剂行业国内领先的科技公司之一。

凭借多年病毒蛋白研究技术优势,义翘神州仅用11天就成功表达出新冠病毒重要靶点受体结合域蛋白。为了更好的支持新冠病毒研究,义翘神州已开发生产近百种新冠病毒相关科研试剂,包括重要靶点抗原蛋白、中和抗体、抗原蛋白检测试剂盒等。义翘神州还可以提供新冠假病毒中和检测服务,可用于筛选出具有中和活性的抗体或血清,支持在细胞水平上的评估。目前,这些科研试剂已被全球数百家研究机构用于疫苗和抗体开发及基础机制研究。

风险提示:丁香通仅作为第三方平台,为商家信息发布提供平台空间。用户咨询产品时请注意保护个人信息及财产安全,合理判断,谨慎选购商品,商家和用户对交易行为负责。对于医疗器械类产品,请先查证核实企业经营资质和医疗器械产品注册证情况。
- 作者
- 内容
- 询问日期
 文献和实验
文献和实验2, van Tol, S; et al. Accurate Serology for SARS-CoV-2 and common Human Coronaviruses using a Multiplex Approach. Emerging microbes & infections, PMID: 32819220
3, Cazares, LH; et al. Development of a parallel reaction monitoring (PRM) mass spec-trometry-assay for the detection of SARS-CoV-2 spike glycoprotein and nucleoprotein. Analytical chemistry, PMID: 32966064
4, Lu, S; et al. The immunodominant and neutralization linear epitopes for SARS-CoV-2. Cell reports, PMID: 33503420
5, Anderson, EM; et al. Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. medRxiv : the preprint server for health sciences, PMID: 33200143
6, Piepenbrink, MS; et al. Therapeutic activity of an inhaled potent SARS-CoV-2 neutralizing human monoclonal antibody in hamsters. Cell Reports Medicine, PMID: 33649747
7, Emeribe, AU; et al. Humoral immunological kinetics of severe acute respiratory syndrome coronavirus 2 infection and diagnostic performance of serological assays for coronavirus disease 2019: an analysis of global reports. International health, PMID: 33620427
8, Yuen, RR; et al. Novel ELISA Protocol Links Pre-Existing SARS-CoV-2 Reactive Antibodies With Endemic Coronavirus Immunity and Age and Reveals Improved Serologic Identification of Acute COVID-19 via Multi-Parameter Detection. Frontiers in immunology, PMID: 33897682
9, Mantus, G; et al. Evaluation of Cellular and Serological Responses to Acute SARS-CoV-2 Infection Demonstrates the Functional Importance of the Receptor-Binding Domain. Journal of immunology (Baltimore, Md. : 1950), PMID: 33952616
10, Maghsood, F; et al. Differential Antibody Response to SARS-CoV-2 Antigens in Recovered and Deceased Iranian COVID-19 Patients. Viral immunology, PMID: 34534012
11, Agarwal, DK; et al. Highly sensitive and ultra-rapid antigen-based detection of SARS-CoV-2 using nanomechanical sensor platform. Biosensors & bioelectronics, PMID: 34583103 1
2, Díez, JM; et al. Anti-SARS-CoV-2 hyperimmune globulin demonstrates potent neutralization and antibody-dependent cellular cytotoxicity and phagocytosis through N and S proteins. The Journal of infectious diseases, PMID: 34693968 1
3, Verma, A; et al. Monoclonal antibodies protect aged rhesus macaques from SARS-CoV-2-induced immune activation and neuroinflammation. Cell reports, PMID: 34706272 1
4, Warner, BM; et al. Intranasal vaccination with a Newcastle disease virus-vectored vaccine protects hamsters from SARS-CoV-2 infection and disease. iScience, PMID: 34632328 1
5, Langellotto, F; et al. A Modular Biomaterial Scaffold-Based Vaccine Elicits Durable Adaptive Immunity to Subunit SARS-CoV-2 Antigens. Advanced healthcare materials, PMID: 34605223 1
6, Zhong, C; et al. Mucosal vaccination induces protection against SARS-CoV-2 in the absence of detectable neutralizing antibodies. NPJ vaccines, PMID: 34845215 1
7, López-Muñoz, AD; et al. Cell Surface SARS-CoV-2 Nucleocapsid Protein Modulates Innate and Adaptive Immunity. bioRxiv : the preprint server for biology, PMID: 34931195 1
8, Li, M; et al. Longitudinal immune profiling reveals dominant epitopes mediating long-term humoral immunity in COVID-19 convalescent individuals. The Journal of allergy and clinical immunology, PMID: 35074422 1
9, Lee, JH; et al. Rapid Biosensor of SARS-CoV-2 Using Specific Monoclonal Antibodies Recognizing Conserved Nucleocapsid Protein Epitopes. Viruses, PMID: 35215848
20, Quach, HQ; et al. Detection of SARS-CoV-2 peptide-specific antibodies in Syrian hamster serum by ELISA. Journal of immunological methods, PMID: 35439529
21, Wussow, F; et al. COH04S1 and Beta Sequence Modified Vaccine Protect Hamsters From SARS-CoV-2 Variants. iScience, PMID: 35634578 2
2, Nakayama, EE; et al. Anti-nucleocapsid antibodies enhance the production of IL-6 induced by SARS-CoV-2 N protein. Scientific reports, PMID: 35577892 2
3, Wang, Z; et al. Naturally enhanced neutralizing breadth to SARS-CoV-2 after one year. bioRxiv : the preprint server for biology, PMID: 34100013 2
4, Chiuppesi, F; et al. Vaccine-induced spike- and nucleocapsid-specific cellular responses maintain potent cross-reactivity to SARS-CoV-2 Delta and Omicron variants. iScience, PMID: 35846380 2
5, Hajnik, RL; et al. Dual spike and nucleocapsid mRNA vaccination confer protection against SARS-CoV-2 Omicron and Delta variants in preclinical models. Science translational medicine, PMID: 36103514 2
6, Samanovic, MI; et al. Vaccine-Acquired SARS-CoV-2 Immunity versus Infection-Acquired Immunity: A Comparison of Three COVID-19 Vaccines. Vaccines, PMID: 36560562 2
7, Schaefer-Babajew, D; et al. Antibody feedback regulates immune memory after SARS-CoV-2 mRNA vaccination. Nature, PMID: 36473496 2
8, Chiuppesi, F; et al. Stimulation of Potent Humoral and Cellular Immunity via Synthetic Dual-Antigen MVA-Based COVID-19 Vaccine COH04S1 in Cancer Patients Post Hematopoietic Cell Transplantation and Cellular Therapy. Vaccines, PMID: 37766168 2
9, Lin, C; et al. Next-Generation Rapid and Ultrasensitive Lateral Flow Immunoassay for Detection of SARS-CoV-2 Variants. ACS sensors, PMID: 37675933
30, Taks, EJM; et al. The impact of Bacillus Calmette-Guérin vaccination on antibody response after COVID-19 vaccination. iScience, PMID: 37860692
31, Ivanova, EN; et al. mRNA COVID-19 vaccine elicits potent adaptive immune response without the acute inflammation of SARS-CoV-2 infection. iScience, PMID: 38213787 3
2, Dalapati, T; et al. Immunogenicity of Monovalent mRNA-1273 and BNT162b2 Vaccines in Children Less Than 5 Years of Age. Pediatrics, PMID: 38548700 3
3, Geanes, ES; et al. SARS-CoV-2 envelope protein regulates innate immune tolerance. iScience, PMID: 38827398 3
4, Vos, ERA; et al. SARS-CoV-2 Seroprevalence Trends in the Netherlands in the Variant of Concern Era: Input for Future Response. Influenza and other respiratory viruses, PMID: 38837866 3
5, Van Puyvelde, B; et al. Acoustic ejection mass spectrometry empowers ultra-fast protein biomarker quantification. Nature communications, PMID: 38879593 3
6, Jung, W; et al. SARS-CoV-2 infection prior to vaccination amplifies Fc-mediated humoral profiles in an age-dependent manner. Cell reports, PMID: 39213155 3
7, Wang, C; et al. 3D Film-Like Nanozyme with a Synergistic Amplification Effect for the Ultrasensitive Immunochromatographic Detection of Respiratory Viruses. ACS nano, PMID: 39219487 3
8, Zhang, L; et al. Ultrasensitive Rapid Antigen Test by Geometric Lateral Flow Assays and Highly Doped Upconversion Nanoparticles. Analytical chemistry, PMID: 39374910 3
9, Esen, M; et al. First-in-Human Phase I Trial to Assess the Safety and Immunogenicity of an Orf Virus-Based COVID-19 Vaccine Booster. Vaccines, PMID: 39591190
40, Hofstee, MI; et al. High SARS-CoV-2 antibody levels after three consecutive BNT162b2 booster vaccine doses in nursing home residents. Immunity & ageing : I & A, PMID: 39748353
41, Neville, AJ; et al. A selective C5a-derived peptidomimetic enhances IgG response following inactivated SARS-CoV-2 immunization and confers rapid disease resolution following murine coronavirus infection. Frontiers in immunology, PMID: 40255397 4
2, Sadler, CJ; et al. Signal Enhancement in Immunoassays via Coupling to Catalytic Nanoparticles. ACS sensors, PMID: 40390533 4
3, Bean, DJ; et al. Endemic coronavirus infection is associated with SARS-CoV-2 Fc receptor-binding antibodies. Journal of virology, PMID: 40387363 4
4, Kim, MJ; et al. Development of a time-resolved fluorescence-based lateral flow immunoassay for rapid and sensitive diagnosis of Middle East respiratory syndrome. Scientific reports, PMID: 40715253 4
5, Mak, WA; et al. Seasonal Coronavirus-Induced Immunological Imprinting and Previous Herpesvirus Infections in Patients With Long COVID. Journal of medical virology, PMID: 40884088 4
6, Reguzova, A; et al. Heterologous prime-boost vaccination with VLA2001 and an ORFV-based vector enhances spike- and nucleocapsid-specific immunity in mice. Frontiers in immunology, PMID: 41050708 4
7, Jiao, C; et al. Field-Deployable Immuno-Solid-Phase Microextraction Coupled with Photothermal Imaging for Rapid Pathogen Surveillance in Environmental and Clinical Matrices. Analytical chemistry, PMID: 40932402
His 标签蛋白纯化效果不理想,宝宝心里苦呀。今天,小编来跟大家一起找找原因。 纯化所得组分中没有收集到重组的His标签蛋白。该问题主要可以分为以下两个方面: 1、His标签蛋白没有结合到填料上就流穿了 原因一 超声的功率不对。超声功率过高,容易导致蛋白炭化;功率过低,蛋白释放不出来。 建议 改变超声功率,同时可以在超声前加入溶菌酶。 原因二 结合缓冲液条件不合适。 建议 检查结合缓冲液中是否有影响结合的因素,如:金属离子螯合剂、强还原剂、过高的咪唑浓度。同时,优化缓冲液的pH、盐
Cell Death Differ:中大五院黄曦团队发现新冠病毒引起肺损伤的分子机制
近期,中山大学附属第五医院黄曦团队报道了 SARS-CoV-2 的 membrane (M) 蛋白通过靶向并抑制胞内 BOK 的泛素化降解,激活肺上皮细胞线粒体凋亡途径而引起肺损伤,该发现为 COVID-19 的靶向治疗提供了潜在靶点。 图片来源:Cell death & differentiation SARS-CoV-2 感染已经导致全球数以百万人的死亡,其中大部分死亡患者出现呼吸困难,肺部出现大量凋亡的细胞和渗漏的粘液,已有研究证实 SARS-CoV-2 感染引起肺细胞凋亡参与上述
重组蛋白纯化基本原则蛋白的分离纯化是生物工程下游阶段一个比较重要的部分,尤其在基因工程重组蛋白的分离纯化中,上游过程的许多因素会直接影响到下游蛋白的分离,充分利用上游对下游的影响,对蛋白的纯化做一个全面的考虑和整体的设计。是否带有亲和标签,His标签,GST标签等,不同的亲和标签选择不同的纯化方案;可能是可溶性表达,可能形成包涵体,可溶性的蛋白往往需要复杂的纯化步骤,而包涵体易于分离,纯度较高,但回收具有生物活性的蛋白却变的相当困难,需要对聚集的蛋白进行变复性,通常活性蛋白的得率比较低;是否










