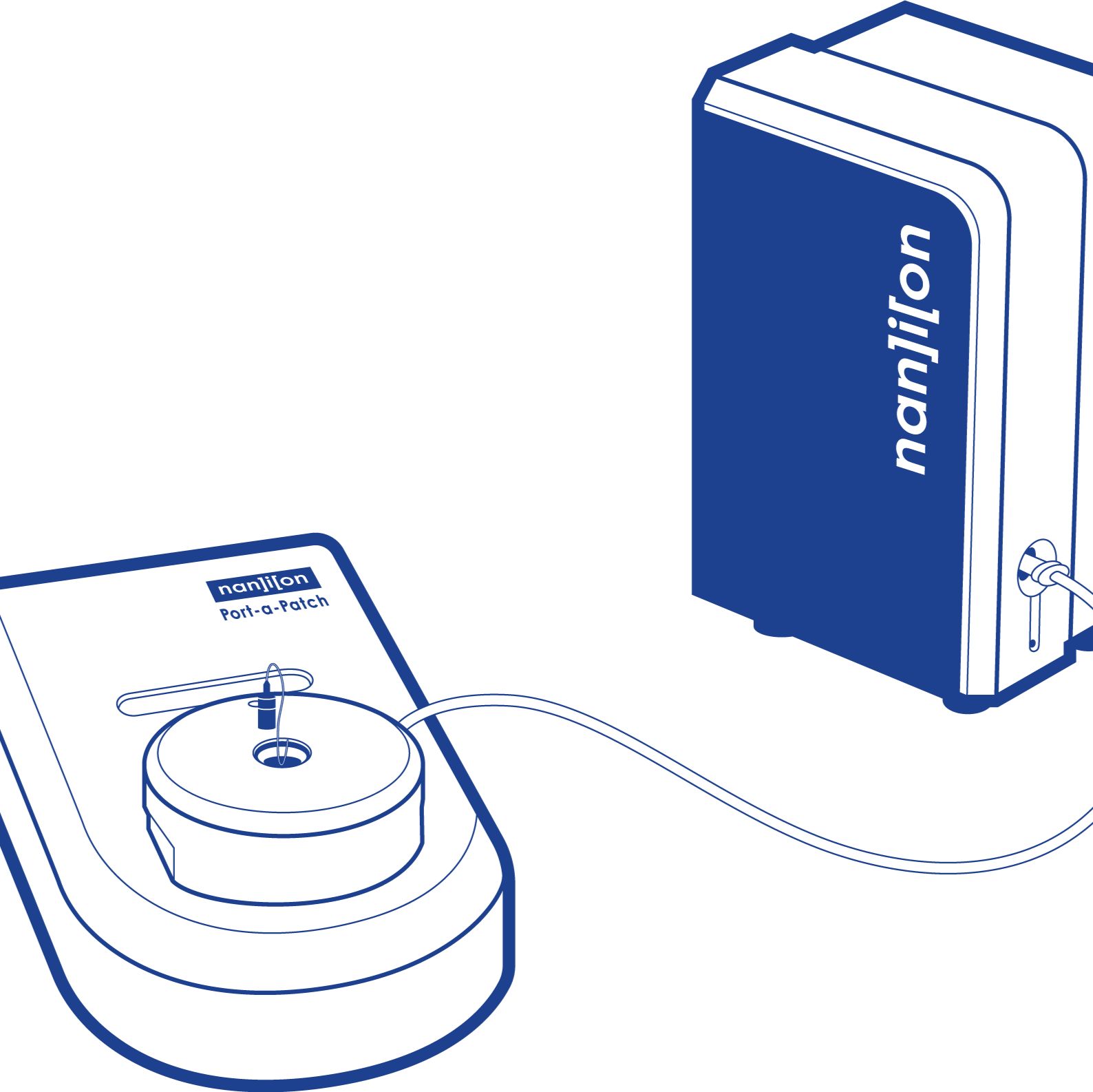
单通道全自动膜片钳Port-a-Patch
- 询价
- nanion
- 德国
- NPC-1
- 2025年07月16日
万千商家帮你免费找货
0 人在求购买到急需产品
- 详细信息
- 询价记录
- 文献和实验
- 技术资料
- 库存:
大量
- 供应商:
德国耐尼恩Nanion
- 现货状态:
有货
- 保修期:
一年
- 规格:
Port-a-Patch
Port-a-Patch是一种集成小型化膜片钳系统,使用户能够快速生成高质量的数据,而无需考虑经验。这使得它成为学习膜片钳电生理学的理想工具。在其小巧紧凑的外观背后,隐藏着复杂的技术,可在大多数常见的记录配置中产生具有千兆级密封的高质量测量和高成功率。它的强度和灵活性体现在多个附加组件中,为用户提供了离子通道研究的强大工具。
使用Port-a-Patch非常简单,用户只需将溶液和细胞添加到一次性记录芯片上,然后通过计算机控制的泵将细胞自动捕获并密封。显微镜或微操作器是不需要的。
内部灌注和温控外部灌注等附加组件,以及进行电流钳位和双分子层实验的能力,极大地拓宽了科学研究可能的实验范围。
该系统由Port-a-Patch记录单元、吸力控制单元、HEKA放大器和计算机组成。如果放大器已经可用,在大多数情况下,这可以与Port-a-Patch系统集成。进一步的附加组件可用于特定的分析,如显微镜载玻片,温度控制,自动灌注系统,压力控制和光激活。
Port-a-Patch是一个小型且易于使用的完整膜片钳设置,具有多个可用的附加组件,以确保您获得适合应用程序的正确配置。
Port-a-Patch有以下一些特点:
- 外液和内液灌流功能
- 支持温度控制
- 支持电流钳和电压钳
- 支持全细胞记录与穿孔膜片钳记录
- 严格质控、自主生产的单孔与多孔芯片
- 支持串联电阻Rs补偿
- 支持无限次灌流
- 电压与配体门控离子通道研究
- 可记录细胞系、原代细胞和干细胞
- 易学易用
- 可在实验过程中随时调整参数,实现快速高效的实验开发
附件与功能
Port-a-Patch平台
Port-a-Patch平台是一个完整的半自动膜片钳系统,用于一次记录单个细胞。该系统包含一个HEKA EPC10放大器,用于细胞捕获和密封的软件,数据采集软件,NPC-1芯片和一个试剂启动器套件,以确保您从第一天开始就得到结果。
Port-a-Patch温控外部灌注系统
Port-a-Patch的外部灌注系统包括一个计算机控制的磁夹管面板和一个温度范围为10 - 50°C的专用温度可控流室(TC流室)。灌注面板可处理多达八种不同的溶液。解决方案既可以手动切换,也可以通过软件切换。后者允许同步数据采集与溶液交换,以及标签收集的痕迹与相关信息,如化合物和浓度应用。
Port-a-Patch内部灌注系统
Nanion的平面膜片钳芯片提供了独特的可能性,灌注膜的细胞内一侧。这方面是利用Port-a-Patch的内部灌注系统插件。使用Nanion的灌注面板和专门设计的内部灌注室,可以在细胞内灌注多达八种不同的溶液。这允许多种浓度的化合物作用于离子通道的胞质部分被应用。第二信使和代谢物也可以在内部添加,以研究离子通道功能是如何受到影响的。溶液在几秒钟内交换,离子通道调制可以在溶液交换过程中持续监测。内部灌注系统可以手动操作,也可以完全由计算机控制。这样,数据采集可以与溶液交换同步,并自动跟踪标记化合物信息。
Port-a-Patch SOL
Port-a-Patch SOL是一种专门为Port-a-Patch设计的保护盖,最多可以连接三个光源。Port-a-Patch SOL非常适合在Port-a-Patch记录过程中进行光学刺激。Port-a-Patch SOL Kit由一个光学盖子和一个大功率LED光源组成。光源既可以手动控制,也可以由放大器软件控制。另外,我们提供的SOL插件没有光源,因此您可以自由使用自己的光源。
PatchContol: Port-a-Patch的简单易用的软件
PatchControl是一个为用户编写的软件程序。可以加载和修改预先编写的协议,允许完全自动化捕获、密封和获取整个单元配置的整个过程。然而,因为我们知道有时你只是想自己控制事情,协议可以随时中断,手动控制吸力。
通过与电生理学软件的创新通信,PatchControl可以读取重要参数,如膜电阻、全细胞电容和串联电阻,并相应地执行预先编程的步骤。诸如吸力强度和持续时间、电压和膜标准等参数可以设置和更改,以获得实验室中常规使用的细胞的最佳参数。不仅如此,复杂的宏可以很容易地实现,从简单的增益变化到复杂的灌注例程,都可以编程并自动执行。
PatchControl软件可以让您的膜片钳的生活更轻松,从自动细胞捕获到自动压力控制,PatchControl软件可以做到这一切!
电生理数据采集
一个完整的Port-a-Patch系统带有预安装和配置的HEKA PatchMaster软件。这是一个功能强大的电生理学数据采集软件,用于手动膜片钳实验。手动膜片钳的电压协议可以加载到Port-a-Patch上的PatchMaster。在PatchMaster中可以选择各种各样的分析参数,并在实验过程中自动显示和导出在线分析值。Port-a-Patch与其他电生理学数据采集程序兼容,例如pClamp或WinWCP,以及手动膜片钳设置的电压协议,简单地传输并在Port-a-Patch上使用。
NPC-1芯片
NPC-1芯片是Nanion Technologies公司为Port-a-Patch开发的专利创新产品。它是在Nanion总部内部生产和质量保证的,并从慕尼黑运往我们的国际客户。不同类型的NPC-1芯片可供选择,应根据细胞大小和应用程序。
描述和材料
带有微米大小贴片孔径的平面硼硅酸盐玻片
硼硅酸盐层纳入螺旋帽,便于操作
实验之间最少的准备
可提供的芯片类型
- "NPC-1, 1-2 MOhm": (Order # 061101)
- "NPC-1, 2-3.5 MOhm": (Order # 061102)
- "NPC-1, 3-5 MOhm": (Order # 061103)
- "NPC-1, 5-10 MOhm": (Order # 061104)
- "NPC-1, 8-12 MOhm": (Order # 061105)
- "NPC-1, 10-15 MOhm": (Order # 061106)
其他孔径也可以定制!
数据与应用:
AMPA Receptor (GluA2) - Reproducible Responses
GluA2 reproducibly recorded on the Port-a-Patch. L-glutamate was applied for 500 ms and this was repeated six times in the same cell showing reproducible responses.
AMPA Receptor (GluA2) - Fast Perfusion with the Port-a-Patch
The AMPA receptor (GluA2) was activated by increasing concentrations of glutamate on the Port-a-Patch. L-glutamate was applied for approximately 500 ms in increasing concentrations (left) and a cumulative concentration response curve for glutamate was constructed for 8 cells (right). The fast activation of GluA2 could be captured at higher concentrations (inset; 1 mM).
CaV2.2 - Current-to-Voltage Relationship
Cells were kindly provided by Millipore.
Representative current responses of an individual HEK-293 cell expressing CaV2.2 to a standard voltage protocol. Average current-voltage relationship (n = 10). The error bars reflect the standard error of the mean (S.E.M.).
hERG - Pharmacological Experiments at 35 °C
A full dose response curve of quinidine acting on the hERG channel was obtained at physiological temperature (35 °C). The IC50 for quinidine at physiological temperature was 1.3 ± 0.2 μM (n = 5), similar to that obtained at room temperature (1.0 ± 0.03 μM, n = 3).
Raw traces of TRPV1 current responses to voltage ramps from –100 mV up to +100 mV. TRPV1 current at RT (28°C), during two stimulations by application of heated solution (34°C and 36°C), and after cooling down to RT (29°C). The picture on the right-hand side shows the current amplitudes as recorded at -100 mV and +100 mV plotted against time.
KV1.3 - Voltage Induced Membrane Movements Measured with Atomic Force Microscopy
Voltage induced membrane movements of a Jurkat cell. (a) Deflection signal of the cantilever resting on the cell membrane at an indenting force of 1.0 nN. (b) Corresponding whole cell current: The characteristic response of the voltage gated potassium channel KV1.3 in Jurkat cells is observed. (c) Below: Applied pulse protocol.
KV1.3 - Internal Application of TEA and Quinidine
KV1.3 current was blocked by the internal application of increasing concentrations of quinidine (left) or TEA+ (right). For quinidine the IC50 was determined as 15 μM (literature value external application 14 μM) and for TEA+ the IC50 was determined as 0.9 ± 0.3 mM (n = 3) (literature value internal application 0.6 mM).
Gramicidin - Rapid Internal Solution Exchange
Switching of internal solutions during gramicidin recordings from a lipid bilayer was obtained within seconds. A lipid bilayer was formed using giant unilamellar vesicles. Currents were recorded at a holding potential of +150 mV. The internal solution was switched from HCl to KCl, resulting in lower channel conductance.
TRPV1 - Internal Application of Calmodulin
TRPV1 currents were elicited by the external application of 20 μM capsaicin. After capsaicin activation, the currents were partially blocked by the internal application of Ca2+-calmodulin (50 μM Ca2+ / 500 nM CaM). TRPV1 channels were expressed in CHO cells.
KCa1.1 (BK) - Intracellular Second Messengers
Currents mediated by BK channels expressed in CHO cells were studied using the Internal Perfusion System. Currents were elicited by a voltage step from -80 mV to +80 mV before and after adding an internal solution containing a higher concentration of free Ca2+.
Shown are mTRPM7 (HEK293) raw current responses to a voltage ramp voltage procotol (−100 mV to 100 mV over 200 ms) recorded under control conditions and after subsequent internal perfusion with Mg2+ (left). Initally, currents showed some run-up before they became stable. The arrows in the timecourse on the right mark the timepoints for which the raw current traces are shown.
KV1.3 - Continuous Internal Perfusion
KV1.3 currents (blue), endogenously expressed in Jurkat cells, were rapidly blocked by internal perfusion of Cs+ (light blue), and fully recovered after washout with K+ (grey). Internal solution replacement was repeated 19 times and the recording was stable for over 35 minutes, as shown in the lower graph.
Glycine Receptor (GlyRa1) - Pharmacology
Cells were kindly provided by AstraZeneca, Sweden.
The Laminar Flow Chamber can also be used for studying glycine receptors. The pharmacology of the hGlyRα1, expressed in a mouse fibroblast cell line (L-tk), was investigated. The EC50 for glycine was determined as 89 ± 2.7 μM (n = 10) which is in accordance with the literature.
GABAA Receptor (a1b2g2) - Reliable Compound Application
Ligand dependent activation of GABAA receptors can be recorded with the Laminar Flow Chamber with approx. 100 ms time constant for 10 μM GABA. Concentration dependent activation and desensitization of GABAA (α1β2γ2) receptors was obtained by applying 1, 3 and 10 μM GABA for 2.5 s at a time interval of 20 s.
MscL - Pressure Clamp Experiments
The Port-a-Patch and the Suction Control Pro was used to study the effect of pressure on functional MscL protein reconstituted in GUVs. Using symmetrical KCl solutions, MscL gating was observed at -30 mBar and -60 mBar (holding potential + 30 mV), shown as outward currents in the data traces.
Neural Cells (Primary Mouse Stem Cells) - Current Traces
Data courtesy of Dr. Gavin Dawe, National University of Singapore.
Patch clamp recordings from cultured mouse primary neuronal stem cells were made in the whole cell configuration (holding potential −60 mV). Currents were evoked by 500 ms depolarizing voltage pulses, showing outwardly rectifying K+- currents.
Neurons (Primary Hippocampal Granule Cell) - Current Traces
Recordings from acutely isolated hippocampal granule cells show BK- and CaV currents. Whole cell currents were obtained by depolarizing voltage pulses from a holding potential of −80 mV.
NaV1.5 - Accurate Voltage Control
Cells stably transfected with human SCN5A were kindly provided by Millipore.
To perform recordings of fast events, such as the activation and inactivation of Na currents, it is essential to have accurate voltage control. The image shows currents and I/V characteristics of NaV1.5 expressed in HEK293 cells.
Cardiac Action Potentials - hESC-derived Cells
Cells were kindly provided by Geron/GE.
The left picture shows a typical action potential in hESC-derived cardiomyocytes (human embryonic stem cell-derived cardiomyocytes). The traces represent K+- (top left), Ca2+- (bottom left) and Na+-currents (top right). The lower right panel shows the corresponding I/V-relationships.
Cardiac Action Potentials - Cor.At® Cells
Cells were kindly provided by Axiogenesis
Cor.At® cardiomyocytes are derived from mouse embryonic stem (ES) cells. Whole cell currents recorded in voltage clamp mode reveal cardiomyocyte-typical ion channels (K+, Ca2+ and Na+). Traces on the lower left show prolongation of the action potential upon application of Dofetilide (1 μM).
TRPV1 data were kindly supplied by Dr. David Cohen, Oregon Health and Service University, Portland, OR, USA.
TRP channels recorded in HEK293 cells. Menthol (dark blue) activated the TRPM8 channels (shown on the left) and could be inhibited by genistein (blue). TRPV1 channels expressed in HEK293 cells (right) were stimulated by application of capsaicin.
External application of 300 µM menthol activated the TRPM8 channels expressed in HEK293 cells.
CaV3.2 - T-Type Calcium Channels
Cells were kindly provided by Cytomyx Millipore.
Shown are raw current traces (top left) and average peak current data (top right) of the current voltage relationship of CaV3.2 (T-type Ca2+- Channel) stably expressed in HEK293 cells. Activation and Inactivation plots were constructed (bottom). Half-activation and half-inactivating potentials were determined as -32 mV and -65 mV, respectively
Cells were kindly provided by Cytomyx Millipore.
Shown are raw current traces (top) and average dose response curves (bottom) of CaV3.2 (T-type Ca2+- Channel) block by compounds as indicated. CaV3.2 is stably expressed in HEK293 cells. IC50s were 863 nM (Mibefradil), 27 μM (Nifedipine) and 52 μM Amiloride
hERG - Good Results with "Sticky Compounds"
Cells were kindly provided by Cytomyx/Millipore.
Sticky compounds, such as some hERG blockers, exhibit expected IC50 values with the Port-a-Patch. The concentrations of the half maximal block were: 1.27 nM (astemizole), 8.9 nM (cisapride), 163.7 nM (flunarizine) and 11.0 nM (terfenadine).
Bilayer Recordings - Low Noise
Typical recording of the Irms noise level of a bilayer (DPhPC) on the Low Capacitance Holder. Typical values are 220 fA
(10 kHz filter frequency, 50 kHz sampling rate. Cf. 2.3 pA RMS on the standard Port-a-Patch).
Cells were kindly provided by Cytomyx/Millipore.
The image shows example traces for hERG mediated currents at 25 ± 2 °C (black) and 35 ± 2 °C (blue). The peak current amplitude was increased at 35 °C, and the rise time and decay time constants were faster at physiological temperature compared with those obtained at room temperature.
Protoplasts (Mesophyll Tabacco Leaves) - Hyperpolarization-Activated Inward Currents
Data were kindly provided by Berghöfer; Eing; Flickinger; Frey (Forschungszentrum Karlsruhe, Germany).
Mesophyll protoplasts are enzymatically isolated from tobacco leaves. Shown are whole cell recordings which demonstrate the hyperpolarization-activated inward currents in the protoplasts. A voltage ramp from −40 mV to −120 mV was applied.
KV1 (Shaker-Relared Potassium Channels) - Block of Mutated Channel
Data were kindly provided by Dr. Kenton Swartz, NIH, Bethesda, USA.
Block of Shaker-IR by Agitoxin2. Shown are current recordings from HEK293 transiently expressing Shaker-IR. Currents were elicited by a 500 ms step to +50 mV from a holding potential of -80 mV. K+ gradients are the same as for the IV curve experiment shown above. 200 nM Agitoxin2 almost fully blocks the Shaker K+ current.
Alpha-Hemolysin - DNA Translocation
Data were kindly supplied by Prof. Fritz Simmel, Technical University of Munich, Munich, Germany.
Reconstituted Alpha hemolysin channels are constantly open at positive and negative membrane potentials. Gating is observed as a result of the passage of a single stranded DNA molecule through the pore. Recordings were performed on the Port-a-Patch.
TRPM8 - Bilayer Recording
Data kindly provided by Dr. Zakharian and Dr. Rohac From UMDNJ New Jersey Medical School, Newark, NJ 07103, USA
Shown are single channel events of TRPM8 reconstituted in a planar lipid bilayer. The recordings were made with the with the Port-a-Patch. The channel was activated by PIP2 and methanol. Currents were recorded at 100 mV.
Cytolysin (Bacterial) - Bilayer Recordings
Recordings were kindly supplied by ITC & CNR-Istituto di Biofisica, Trento, Italy.
Traces were recorded in 100 mM KCl, 10 mM Hepes, 0.1 mM EDTA, pH 7 at -40 mV. Lipid: Diphtanoyl-PC.
Alamethicin - Bilayer Recordings
Data were kindly provided by M. Sondermann/Prof. Behrends, Univ. Freiburg.
Alamethicin is a channel forming peptide and, when patch clamped, reveals multiple non-equidistant conductance levels due to formation of alamethicin oligomers in the bilayer. Alamethicin single channel conductances. Recordings from a GUV prepared bilayer in 85 mM KCl at -140 mV.
Gramicidin - Single Channel Analysis
Recordings were kindly supplied by Tohoku University, Tohoku, Japan.
Plotting the current amplitude vs. the voltage reveals conductances of 94.88 pS and 28.28 pS, which correspond to two different gramicidin derivates present in the bilayer. Traces were recorded in 100 mM HCl at the indicated potentials. Clearly two gramicidin derivates (94.88 pS and 28.28 pS) can be distinguished.
Mitochondria - Cell Attached Recordings
Mitochondria were isolated from rat cardiac tissue. The patch clamp measurements were performed in the cell attached configuration with an Axopatch 200B. Single channel currents were elicited by a voltage ramp from 0 mV to -80 mV. Due to the cell attached configuration inward currents are displayed as a positive current.
Chloride Channels - Lysosomes Recordings
Data are taken from Schieder M. et al., The Journal of Biological Chemistry, 2010, 285(28), 21219-21222.
Typical chloride currents in four isolated lysosomes.Chloride currents were determined as difference currents between IV curves recorded in the absence and presence of extralysosomal high (64 mM) Cl-. On the left a sketch is illustrating the methodology.
Erythrocytes - Single Channel Analysis
Erythrocytes lack mitochondria and nuclei and consist mainly of hemoglobin. The membrane contains different ion channels, for example, a Ca2+-activated K+ channel and a volume-sensitive Na+/K+ pump. Here, single channel activity recorded from an erythrocyte in the cell attached configuration is shown.
KCa1.1 (BK) - Single Channel Recordings
Single channel recordings of BK channels as recorded from CHO cells in the cell attached mode at +60 mV (top), +40 mV (middle) and 0 mV (lowest trace).
Sonoporation Technology - Contrast Agent Combined with Ultrasound
Data were kindly provided by Dr. Cheri Deng, University of Michigan, USA.
Current response of a non-transfected Chinese hamster ovary (CHO) cell to a train of ultrasound pulses (US) in the absence (A) and presence (B) of contrast agent. The holding potential was -80 mV. Ultrasound signals had a centre frequency of 5 MHz. Pulses of 50 cycles were repeated for 2 s every 10 ms.
BY2 Protoplasts - Current Traces
Tobacco (Nicotiana tabacum L. cv Bright Yellow 2 [BY2]) cells are the most widely used plant cell culture. Depolarisation-activated outward currents in BY2 cell protoplasts. Shown are whole cell current in response to voltage steps (left) and the corresponding IV curve (right). From a holding potential of −40 mV, 500 ms voltage steps were applied from −60 mV to 60 mV in 20 mV increments, with 3 s intervals.
Cardiomyocytes (Ventricular Myocytes) - Recordings
Thanks to S. Rakovic and D. Terrar from University of Oxford, for preparing the cardiomyocytes used in these experiments.
Ca2+ current recorded from a ventricular myocyte. Raw current voltage relationship and peak current data are plotted (top). From the IV-plot the half-activating voltage was determined as -16 mV. The current was later blocked by the L-type Ca2+ channel blocker, nifedepine (10 µM). Currents were elicited by stepping to +10 mV for 200 ms from a holding potential of -40 mV every 5 seconds.
Endoplasmatic Reticulum Membrane Protein Translocator (Sec61) - Single Channel recordings
Data were kindly provided by A. Cavalié, M. Jung, R. Zimmermann, Universität des Saarlandes, Homburg, Germany.
Microsomes were fused with GUVs within the VesiclePrepPro. The resulted Proteoliposomes formed a bilayer onto the NPC-1 Chip of the Port-a-Patch. Nice single channel openings could be recorded in symmetrical KCl solution applying a ramp from -110 mV to +100 mV for 400 ms.
TRPV1 - External Perfusion of Capsaicin
Data were kindly provided by David Cohen, Oregon Health & Science University, Portland, USA.
Whole cell current responses from HEK 293 cells transiently expressing TRPV1 to a ramp voltage protocol (‑100 mV to +100 mV). Capsaicin at a concentration of 2 μM reversibly activated the channel.
Cells were kindly supplied by Evotec AG, Hamburg, Germany
Shown are raw current traces from 1321 N1 cells expressing P2X3 receptors demonstrating the reproducability of the whole cell responses. Currents were activated by 100 ms application of 10 µM ATP. The waiting time between sweeps was 120 s (holding −60 mV).
NBC1 (Electrogenic Na+/HCO3- Cotransporter) - Current responses
Data were kindly provided by Ira Kurtz, UCLA, USA.
Recordings from HEK 293 cells expressing the electrogenic Na+/bicarbonate cotransporter (NBC1). Current responses to 200 ms voltage ramps in high external Na+ initially after establishment of the whole cell configuration (control), after application of HCO3- (bicarbonate), and after wash-out (wash-out).
Kir Channels - Response to Different External Potassium Concentrations
Current response in RBL cells to a voltage ramp from –150 mV to +80 mV in the presence of a low and a high external K+ concentration (left). Voltage dependent block of the inward K+ current after the external additon of 50 µM Ba2+ (middle). Same block in response to a continuous voltage step to –150 mV (right) from 0 mV. Low K+ (4.5 mM K+), high K+ (143 mM K+).
KV1 (Shaker-related Potassium Channels) - Mutation without Inactivation
Data were kindly provided by Dr. Kenton Swartz, NIH, Bethesda, USA.
Here we show recordings from HEK293 cells transiently expressing a Shaker K mutant that has its inactivation removed (Shaker-IR). The holding potential was -80 mV. The current-voltage relationship of the tail currents reveals a half-activating potential of -27 mV.
IMR32 Differentiated Cells - Sodium and Potassium Currents
Whole cell current responses of undifferentiated (left) and for 12 days differentiated (right) IMR32 cells to a voltage step protocol (holding −90 mV, stepping for 20 ms from −60 mV to +60 mV in 20 mV increments) are shown. The change in current shape indicating increased expression of KV and NaV are apparent.
Potassium Channels - Currents Measured in Differentiated IMR32 Cells
Whole cell current responses of undifferentiated (a) and for 12 days differentiated (b) IMR32 cells to a voltage step protocol (holding −90 mV, stepping for 500 ms from −60 mV to +60 mV in 10 mV increments) are shown. In (c) and (d) the corresponding current voltag relationships are shown (closed circles). Open circles are the correspondig current voltage relationships after the cells have been exposed to internal Cs+.
EAG1 (hKv10.1) - Internal Application of TEA
Data were kindly provided by Walter Stühmer, MPI for exp. Med., Göttingen.
Block of hEAG whole cell currents after the internal application of 200 µM TEA.
Perforated and conventional whole cell configuration derived hKV1.3-currents. Voltage-dependence was shifted significantly to more negative potentials in the whole cell configuration compared to the perforated whole cell configuration. Whole cell currents after a conventional membrane breakthrough (right) and perforated whole cell currents (left).
CNG - cAMP-Regulation of a Plant Ion Channel
Data were kindly provided by A. Kugler & P. Dietrich, Univ. Erlangen, Germany.
The image shows internal cAMP-activation of a transiently expressed plant CNG channel and subsequent external block by lanthanum. Activators and blockers were manually added to the intracellular and extracellular sides of the membrane.
发表文献:
2021 - Translational Studies on Anti-Atrial Fibrillatory Action of Oseltamivir by its in vivo and in vitro Electropharmacological Analyses
2021 - The mechanism of non-blocking inhibition of sodium channels revealed by conformation-selective photolabeling
2021 - The human TRPA1 intrinsic cold and heat sensitivity involves separate channel structures beyond the N-ARD domain
2021 - Taspine is a natural product that suppresses P2X4 receptor activity via phosphoinositide 3-kinase inhibition
2021 - Study of Anti-Inflammatory and Analgesic Activity of Scorpion Toxins DKK-SP1/2 from Scorpion Buthus martensii Karsch (BmK)
2021 - Structure, gating and interactions of the voltage-dependent anion channel
2021 - Norfluoxetine inhibits TREK-2 K2P channels by multiple mechanisms including state-independent effects on the selectivity filter gate
2021 - Multiple Mechanisms Underlie State-Independent Inhibitory Effects of Norfluoxetine on TREK-2 K2P Channels
2021 - L-carnitine suppresses transient receptor potential vanilloid type 1 activity and myofibroblast transdifferentiation in human corneal keratocytes
2021 - Gating the channel pore of ionotropic glutamate receptors with bacterial substrate binding proteins
2021 - An antibiotic-resistance conferring mutation in a neisserial porin: Structure, ion flux, and ampicillin binding
2021 - An advanced automated patch clamp protocol design to investigate drug – ion channel binding dynamics.
2021 - Active components of Bupleurum chinense and Angelica biserrata showed analgesic effects in formalin induced pain by acting on Nav1.7
2021 - Abnormal podocyte TRPML1 channel activity and exosome release in mice with podocyte-specific Asah1 gene deletion
风险提示:丁香通仅作为第三方平台,为商家信息发布提供平台空间。用户咨询产品时请注意保护个人信息及财产安全,合理判断,谨慎选购商品,商家和用户对交易行为负责。对于医疗器械类产品,请先查证核实企业经营资质和医疗器械产品注册证情况。
- 作者
- 内容
- 询问日期
 文献和实验
文献和实验- 2023- Pharmacology of transient receptor potential cation (TRP) channels using different activation stimuli
- 2015 – Organellar Transporters and Ion Channels – How to access their electrophysiology by using the SURFE2R technology and Planar Patch Clamp
- 2015 – The backstage pass to study your favorite TRP channel
- 2021 – Activation and inhibition of assay-ready TRPA1 and TRPV cells: an automated patch clamp study
- 2020 – Electrophysiological characterization of transport across outer membrane channels from Gram‐negative bacteria in presence of lipopolysaccharides (LPS)
- OmpF – “Lipid Bilayer recordings of OmpF reconstituted in Proteoliposomes “
- MscL – “Lipid bilayer recordings of a mechanosensitive channel, MscL, using Nanion’s pressure clampchannel, MscL, using Nanion’s pressure clamp”
- 2022 – Temporin B Forms Hetero-Oligomers with Temporin L, Modifies Its Membrane Activity, and Increases the Cooperativity of Its Antibacterial Pharmacodynamic Profile
- 2022 – The human TRPA1 intrinsic cold and heat sensitivity involves separate channel structures beyond the N-ARD domain
- 2022 – Cannabinoid non-cannabidiol site modulation of TRPV2 structure and function
- 2022 – Balancing water solubility with membrane permeability in the design of a synthetic ionophore
,给生命科学研究带来了巨大的前进动力。 三、全自动膜片钳技术 膜片钳技术被称为研究离子通道的"金标准"。是研究离子通道的最重要的技术。目前膜片钳技术已从常规膜片钳技术(Conventional patch clamp technique)发展到全自动膜片钳技术(Automated patch clamp technique)。 传统膜片钳技术每次只能记录一个细胞(或一对细胞),对实验人员来说是一项耗时耗力的工作,它不适合在药物开发初期和中期进行大量化合物的筛选,也不适合需要记录大量细胞的基础
神经生物学名词-molecular biology of sodium and potassium channels
Patch clamping 膜片钳 研究单离子通道的电生理方法 通过在玻璃微管和细胞表面形成高阻抗而起作用。电流流过小孔将被记录,这使得流过单离子通道的极小电流被记录。电子使得小孔电压被开关所以电压开关实验也可以进行。 Macroscopic currents 可见电流 Unitary channel current 单通道电流 Mean channel open time 平均
在膜片钳技术的发展过程中,主要形成了四种记录模式,即细胞贴附模式(cell-attached mode或on-cell mode)、膜内面向外模式(inside-out mode)、膜外面向外模式(outside-out mode)、常规全细胞模式(conventional whole-cell mode)和穿孔膜片模式(perforated patch mode),如图11-2所示。根据研究目的和观察内容的不同,可采取相应的记录方法。此外,还有带核膜片记录、人工脂膜











