相关产品推荐更多 >

美天旎Miltenyi货号170-076-306专门培养人T细胞和Treg细胞的培养基TexMACS™ GMP Medium上海睿安生物13611631389
¥4482.71
Miltenyi货号130-045-201人CD8磁珠(human CD8 MicroBeads)上海睿安生物13611631389
¥8030.91
Miltenyi货号130-045-101现货CD4 MicroBeads(human)(人CD4磁珠)13611631389上海睿安生物
¥8030.91
美天旎Miltenyi货号130-097-043人-冻干CD3磁珠(human CD3 MicroBeads)上海睿安生物13611631389
¥8030.91
美天旎Miltenyi货号130-117-044小鼠CD8a(Ly-2)磁珠19121878899上海睿安生物Miltenyi官方正式授权
¥9149.61
万千商家帮你免费找货
0 人在求购买到急需产品
- 详细信息
- 文献和实验
- 技术资料
- 库存:
100⁺盒
- 英文名:
CliniMACS® Tubing Set,CE
- 保质期:
1年
- 供应商:
上海睿安生物13611631389
- 保存条件:
Room Temperature
- 规格:
1 set/盒
美天旎Miltenyi货号200-073-104现货CliniMACS® Tubing Set,CE上海睿安生物13611631389
Specifications for CliniMACS® Tubing Set and Tubing Set LS
Overview
CliniMACS® Tubing Sets consist of preassembled tubing including a pre-column and a separation column. The fluid path of the tubing sets is sterile and non-pyrogenic.
One package unit contains one sealed tubing set for single use.
The performance of the CliniMACS Tubing Sets depends on the individual CliniMACS Separation strategy. For information on respective capacities, refer to the CliniMACS User Manual or contact your local representative.
Please inquire about required CliniMACS System components and accessories.
Detailed product information
Applications
The CliniMACS Tubing Sets for clinical use were developed for the enrichment or depletion of cells from human heterogeneous hematologic cell populations in combination with the CliniMACS System.
Disclaimer
The CliniMACS® System components, including Reagents, Tubing Sets, Instruments, and PBS/EDTA Buffer, are designed, manufactured and tested under a quality system certified to ISO 13485.
In the EU, the CliniMACS System components are available as CE-marked medical devices for their respective intended use, unless otherwise stated. The CliniMACS Reagents and Biotin Conjugates are intended for in vitro use only and are not designated for therapeutic use or direct infusion into patients. The CliniMACS Reagents in combination with the CliniMACS System are intended to separate human cells. Miltenyi Biotec as the manufacturer of the CliniMACS System does not give any recommendations regarding the use of separated cells for therapeutic purposes and does not make any claims regarding a clinical benefit. For the manufacturing and use of target cells in humans the national legislation and regulations - e.g., for the EU the Directive 2004/23/EC ("human tissues and cells"), or the Directive 2002/98/EC ("human blood and blood components") - must be followed. Thus, any clinical application of the target cells is exclusively within the responsibility of the user of a CliniMACS System.
In the US, the CliniMACS CD34 Reagent System, including the CliniMACS Plus Instrument, CliniMACS CD34 Reagent, CliniMACS Tubing Sets TS and LS, and the CliniMACS PBS/EDTA Buffer, is FDA approved as a Humanitarian Use Device (HUD), authorized by U.S. Federal law for use in the treatment of patients with acute myeloid leukemia (AML) in first complete remission. The effectiveness of the device for this indication has not been demonstrated. Other products of the CliniMACS Product Line are available for use only under an approved Investigational New Drug (IND) application, Investigational Device Exemption (IDE) or FDA approval.
CR/GMP products are for ex vivo cell processing only, and are not intended for human in vivo applications. CR/GMP products are designed, manufactured and tested under an ISO 13485 quality management system and are in compliance with relevant GMP guidelines. They are designed following the recommendations of USP <1043> on ancillary materials. The manufacturing and testing of CR/GMP products of biological origin are in compliance with EP chapter 5.2.12 for “Raw materials of biological origin for the production of cell-based and gene therapy medicinal products”.
Figure 1
The CliniMACS® Tubing Set.
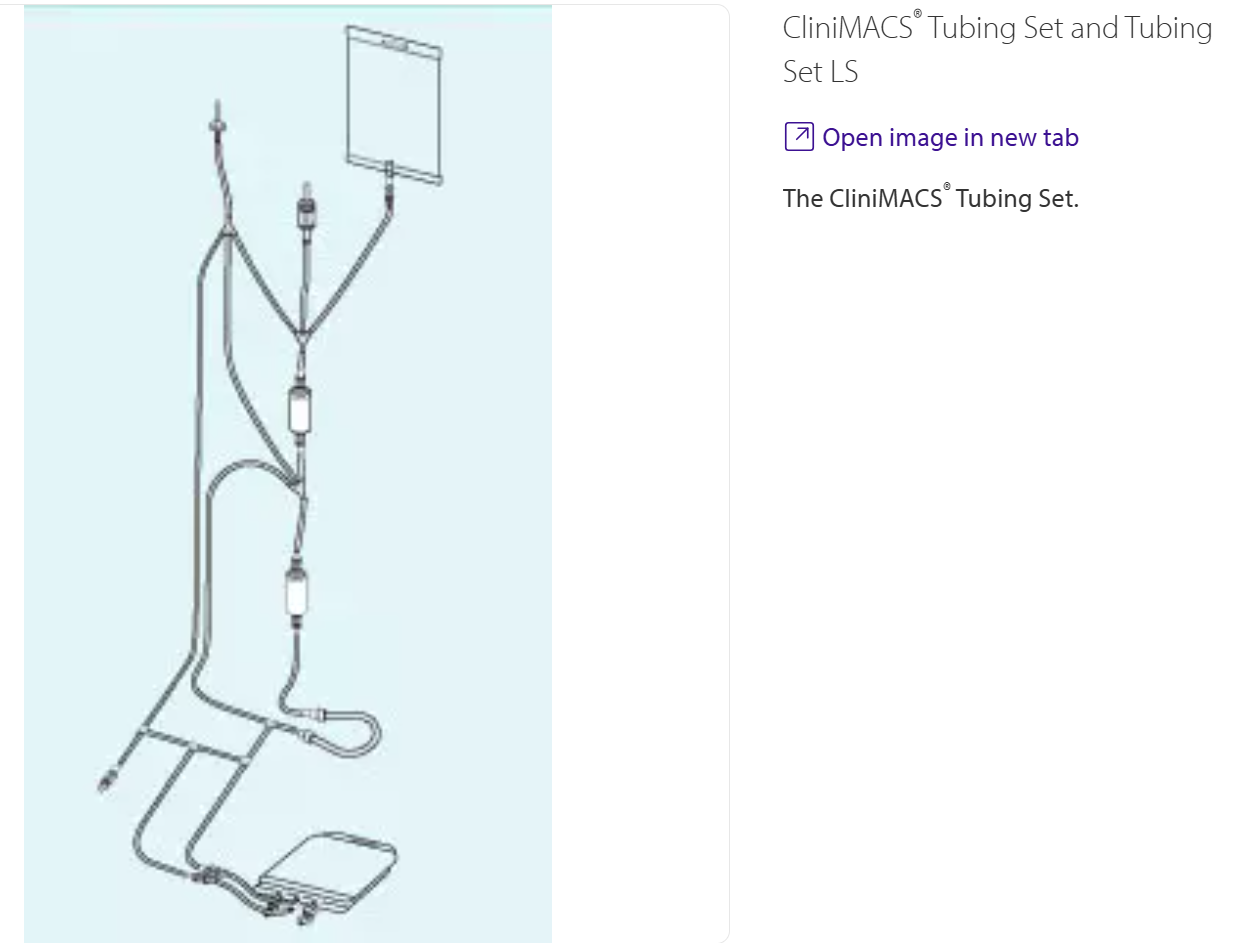
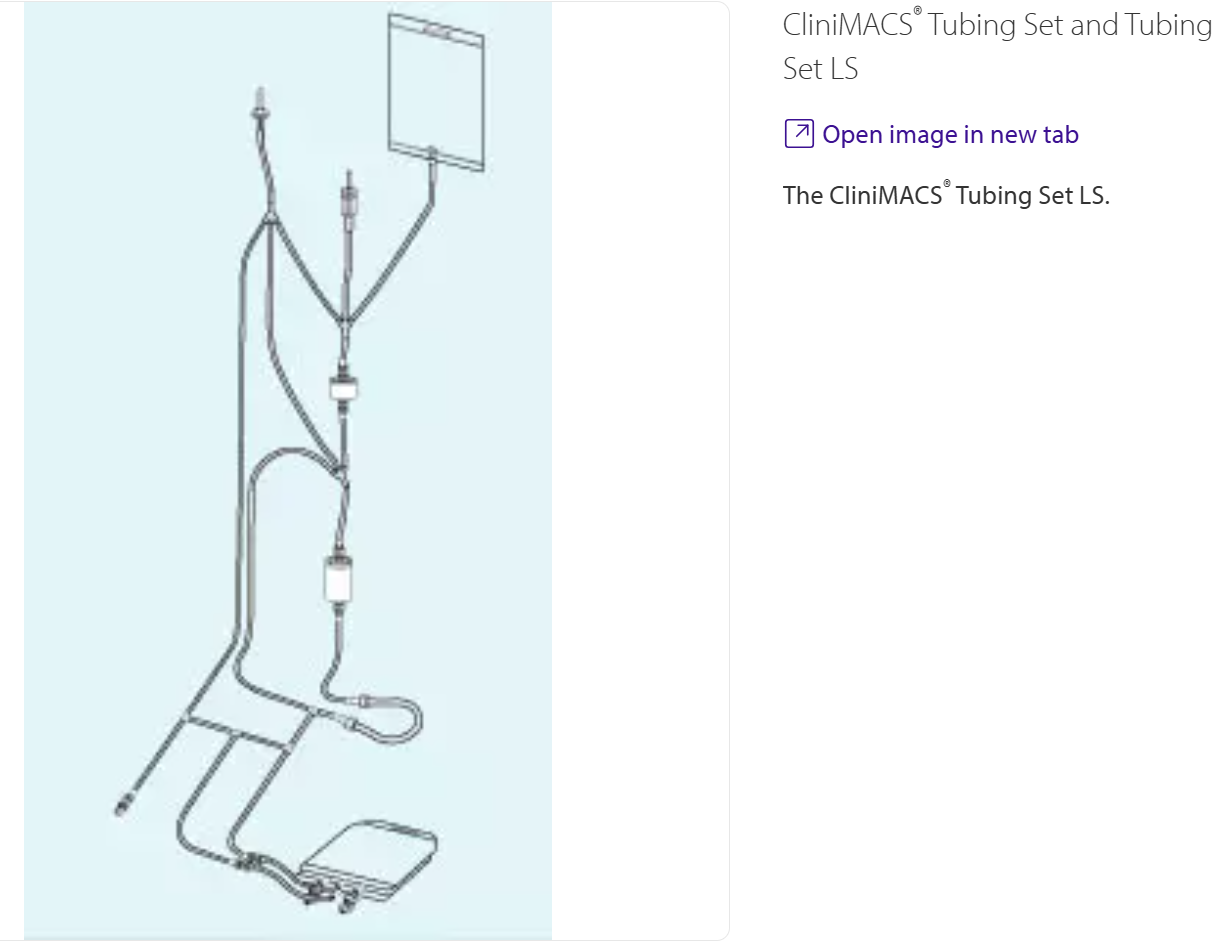
Figure 2
The CliniMACS® Tubing Set LS.

风险提示:丁香通仅作为第三方平台,为商家信息发布提供平台空间。用户咨询产品时请注意保护个人信息及财产安全,合理判断,谨慎选购商品,商家和用户对交易行为负责。对于医疗器械类产品,请先查证核实企业经营资质和医疗器械产品注册证情况。
 文献和实验
文献和实验美天旎Miltenyi货号200-073-104现货CliniMACS® Tubing Set,CE上海睿安生物13611631389
Specifications for CliniMACS® Tubing Set and Tubing Set LS
Overview
CliniMACS® Tubing Sets consist of preassembled tubing including a pre-column and a separation column. The fluid path of the tubing sets is sterile and non-pyrogenic.
One package unit contains one sealed tubing set for single use.
The performance of the CliniMACS Tubing Sets depends on the individual CliniMACS Separation strategy. For information on respective capacities, refer to the CliniMACS User Manual or contact your local representative.
Please inquire about required CliniMACS System components and accessories.
Detailed product information
Applications
The CliniMACS Tubing Sets for clinical use were developed for the enrichment or depletion of cells from human heterogeneous hematologic cell populations in combination with the CliniMACS System.
Disclaimer
The CliniMACS® System components, including Reagents, Tubing Sets, Instruments, and PBS/EDTA Buffer, are designed, manufactured and tested under a quality system certified to ISO 13485.
In the EU, the CliniMACS System components are available as CE-marked medical devices for their respective intended use, unless otherwise stated. The CliniMACS Reagents and Biotin Conjugates are intended for in vitro use only and are not designated for therapeutic use or direct infusion into patients. The CliniMACS Reagents in combination with the CliniMACS System are intended to separate human cells. Miltenyi Biotec as the manufacturer of the CliniMACS System does not give any recommendations regarding the use of separated cells for therapeutic purposes and does not make any claims regarding a clinical benefit. For the manufacturing and use of target cells in humans the national legislation and regulations - e.g., for the EU the Directive 2004/23/EC ("human tissues and cells"), or the Directive 2002/98/EC ("human blood and blood components") - must be followed. Thus, any clinical application of the target cells is exclusively within the responsibility of the user of a CliniMACS System.
In the US, the CliniMACS CD34 Reagent System, including the CliniMACS Plus Instrument, CliniMACS CD34 Reagent, CliniMACS Tubing Sets TS and LS, and the CliniMACS PBS/EDTA Buffer, is FDA approved as a Humanitarian Use Device (HUD), authorized by U.S. Federal law for use in the treatment of patients with acute myeloid leukemia (AML) in first complete remission. The effectiveness of the device for this indication has not been demonstrated. Other products of the CliniMACS Product Line are available for use only under an approved Investigational New Drug (IND) application, Investigational Device Exemption (IDE) or FDA approval.
CR/GMP products are for ex vivo cell processing only, and are not intended for human in vivo applications. CR/GMP products are designed, manufactured and tested under an ISO 13485 quality management system and are in compliance with relevant GMP guidelines. They are designed following the recommendations of USP <1043> on ancillary materials. The manufacturing and testing of CR/GMP products of biological origin are in compliance with EP chapter 5.2.12 for “Raw materials of biological origin for the production of cell-based and gene therapy medicinal products”.
Figure 1
The CliniMACS® Tubing Set.

Figure 2
The CliniMACS® Tubing Set LS.








