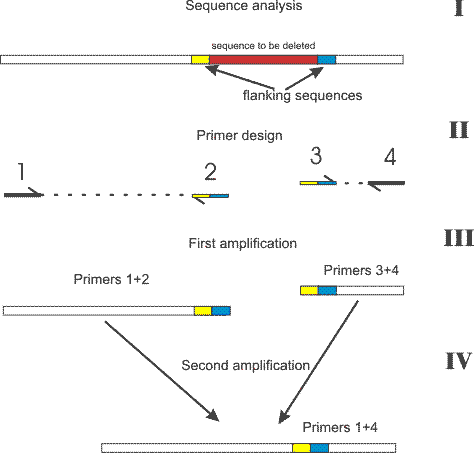
EXO/S1 DELETION SERIES
互联网
2. Prepare one S1 nuclease tube (on ice) for each time point: 15ml S1 Buffer + 0.25U S1/ml (5U).
3. Use 5ml (=0.5mg) digested plasmid (in EB) per time point. Add 4U Exonuclease III/ml reaction mix and incubate @ 37℃ (‰400 digested/min).
4. Remove 5ml aliquots into the S1 nuclease tubes (on ice) at various times. When all time points have been collected, incubate the tubes 30' @ RT.
5. Add 2ml STOP Buffer/tube and incubate 10' @ 70oC.
6. Add: 2.0ml 10X Klenow Mix
+2.0ml 0.125mM dNTPs
+0.5ml Klenow
Incubate 10' @ 37℃.
7. Analyze 10ml on a gel. To the remainder in each tube, add 40ml Ligation Mix + 1ml T4 DNA Ligase. Incubate overnight @ 15℃ (or 2-3h @ RT).
8. Transform 100ml competent cells with 10ml ligation reaction; freeze remainder.
EB
66mM Tris-HCl, pH 8
0.66mM MgCl2
10X Klenow Mix
20mM Tris-HCl, pH 8
100mM MgCl2
S1B
40mM KOAc
300mM NaCl
1.3mM ZnS04
7% Glycerol
Ligation Mix
50mM Tris-HCl, pH7.6
1mM MgCl2
1mM ATP
1mM DTT
5% PEG 6000
S1 STOP 300mM Trizma base
50mM EDTA