Transduction Protocol for Adherent Cell Types
互联网
Purpose
This protocol may be used for the Transduction of adherent cell lines with recombinant Retro-viral vectors (Lenti- and MMLV-~undefined.
~undefinedPlease note that this process is variable and expression efficiency may vary based on cell type. Optimization of conditions may be needed to maximize expression.
下载本文PDF格式: http://www.bbioo.com/Soft/2007/1120.htm

Procedure
24 hours prior to transduction:
- Split target cells into a 6-well tissue culture plate at 2.0-3.0x10
5
per well. The cells should reach the desired confluency of 50-60% the following day for optimal transduction.
Transduction:
- Observe cells under microscope to verify that they look healthy and are at the right confluence.
- In a Tissue Culture Hood, aspirate off cellular media and replace with 1.25ml viral supernatant of desired concentration into each well.
- Add cross linker (polybrene or protamine sulfate) if desired directly to media at a final concentration of 8μg/ml.
- Gently rock the plates to ensure even distribution of virus and linkers
- Incubate cells in incubator at 37℃(32℃ incubator if available) for 8-24 hours
- Replace viral supernatant with cell specific media and return to incubator.
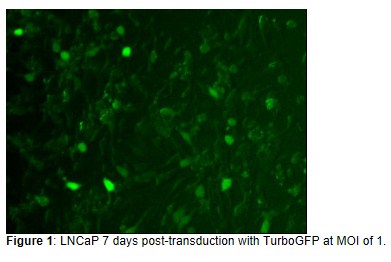
- Analyze trans-gene expression 72 hours post infection. In some instances,trans-gene expression may take up to 7 days post Infection.
Optimization
If needed the following steps may be taken to increase transduction efficiency with retro virus.
1. Cross linkers have been shown to greatly improve transduction efficiency in a wide range of cell types. Polybrene is the most commonly used and seems to be most effective in a wide range of cell types. Protamine Sulfate can also be
effectively used and is less toxic to cells. See table 1 below for set up. The concentration of Polybrene or Protamine may also be raised or lowered if needed.
Table 1. Aliquot the retroviral supernatant into the corresponding wells as described. The media plus localization molecule wells serve as controls to monitor any cell death.

2. Spin-inoculate the cells by placing the plate in a multi-well plate swinging bucket rotor and place in Eppendorf 5810R Centrifuge. Spin the plate at 2500rpm for 90m at 30°C.
3. Incubate the cells at 32oC with 5%CO2 for 16-48h.
Extra Notes
- The splitting of the cells, preparation of infection conditions, infection of target cells,and changing the media must be done using sterile technique in a biological safety cabinet to prevent contamination.
- At any step during the infection, antibiotics may be added to the media or supernatant if contamination is present.
- The starting number of cells may be varied to account for total cell availability. Successful transductions have been carried with as little as 2.0 x 105 cells per well.
- The duration the viral supernatant is on the cells can go from 4 hours to 2 days. We have seen that a longer exposure can produce higher transduction efficiency, although this is proportional to increasing cell death, as VSVG viral envelope and the localization molecules can be toxic. We usually let cells go overnight.
- Multiple infections may be preformed on the same cells to increase the transduction efficiency. To do this allow the 1° infection to stay on the cells overnight, replace the supernatant with media and allow the cells to recover for 24h and then infect again overnight. This cycle may be repeated 3-4 times.
下载本文PDF格式: http://www.bbioo.com/Soft/2007/1120.htm