Mapping the Specificity of SH3 Domains with Phage-Displayed Random-Peptide Libraries
互联网
互联网
相关产品推荐
PIK3R1重组蛋白|PIK3R1 Protein (SH3 domain)
¥2380

Recombinant-Human-Membrane-spanning-4-domains-subfamily-A-member-5MS4A5Membrane-spanning 4-domains subfamily A member 5 Alternative name(s): CD20 antigen-like 2 Testis-expressed transmembrane protein 4
¥10556

T7 Tag Peptide (T7 Tag多肽),≥98%(HPLC),阿拉丁
¥3104.90
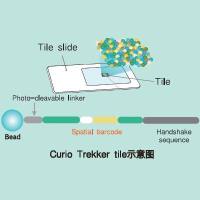
Trekker Single-Cell Spatial Mapping Kit 单细胞空间转录组分析试剂盒
询价

铜,7440-50-8,PrimorTrace™, ≥99.999% metals basis, bars (random sizes),阿拉丁
¥872.90
相关问答

