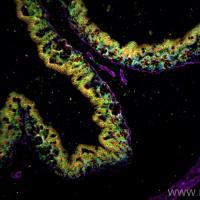
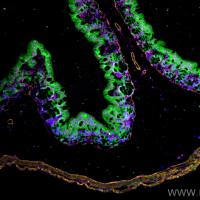
TUNEL labeling
互联网
In Situ Cell Death (Apoptosis) Detection by TUNEL labeling
by Boehringer Mannheim (Catalog No. 1684809), modified by Josiah N. Wilcox and
José C. Rodriguez - Emory University - April 1996
Protocol for Frozen Sections:
- Warm 150ml 4% Paraformaldehyde/1x PBS to RT. Fix slides in it, 20 min., RT.
- 1x PBS rinse, 2 times.
- 1x PBS, 30 min., RT. Begin chilling Triton/SSC on ice.
- 0.1% Triton/ 0.1% Sodium Citrate, 2 min., 4°C.
- All slides: 1x PBS rinse, 2 times (+ 10 min for those non-pos.control slides).
- (Pos. control slide: in DNase I solution (100µl of 200µg/ml), 10 min., RT. 1x PBS rinse, 2 times in a separate container then combine with other slides.)
- Wipe around tissue.
-
Make up negative Control solution (just Label solution containing FITC) and TUNEL solutions at time of use:
A. Remove 100µl from Tube 2 (Label solution) for 2 negative controls (50µl each). Do this even if you are omitting this negative control so that volumes and concentrations will remain consistent for the labeling.
B. Add Total volume (50µl,) of Tube 1 (TdT) + remainder of Tube 2 (450µl).
- Apply 100µl TUNEL reaction mixture (or 100µl Control Label solution for negative control) to each slide.
- Incubate in humid chamber, 60 min., 37°C.
- 1x PBS wash, 3 times.
- Wipe around tissue.
- Apply 100µl anti-FITC-AP conj. ("converter-AP") on each sample.
- Incubate in humid chamber, 30', 37°C.
- 1x PBS wash, 3 times.
- 100mM Tris buffer, pH 8.2, 5 min., RT.
-
Add 50-100µl substrate solution (5-6 drops Vector Blue or Vector Red substrate/per slide):
-
Mix:
5ml 100mM Tris, pH 8.2
1 drop Levamisole
2 drops each of Solution 1, 2, and 3 of either Vector substrate
-
Mix:
- Incubate in absence of light, RT. Vector Blue - 10 min.; Vector Red - 20 min.
- dH2O, 1 time to stop color reaction.
- Counterstain for Vector Blue:
-
Gill's Hematoxylin, No. 2, 5 sec.
Water rinse until clear
Scott's solution, 20 sec.
Water rinse until clear
70% EtOH, 30 sec.
95% EtOH, 2 times, 30 sec. each
100% EtOH, 2 times, 30 sec. each
Histoclear, 1 min.
Histoclear, 1 min.
Coverslip with Accumount medium.
- Counterstain for Vector Red:
-
Gill's Hematoxylin, No. 2, 5 sec.
Water rinse until clear
Scott's solution, 20 sec.
Water rinse until clear
70% EtOH, 30 sec.
95% EtOH, 2 times, 30 sec. each
100% EtOH, 2 times, 30 sec. each
Xylenes, 1 min.
Xylenes, 1 min.
Coverslip with Accumount medium.
- Reagents Needed:
-
3L 1x PBS
10mM Tris-HCl
20 mg/ml Proteinase K
100µl/slide x-phosphate/BCIP or Fast Red substrate
DNase I solution (1mg/ml - 1µg/ml) for Positive control
100mM Tris-Hcl, pH 8.2
4% Paraformaldehyde/PBS, pH 7.4
- Heat 500ml 1x PBS to 65°C.
- Dissolve 20g Paraformaldehyde in the PBS (in a fume hood).
- Filter with Whatman No.1 paper to remove particles; store at 4°C.
0.1% Triton X-100 in 0.1% Sodium Citrate (SSC)
-
Mix:
2ml 20x SSC
400µl Triton X-100
398ml H2O
100mM Tris-Hcl, pH 8.2