SafetyTector™病毒灭活稀释液
研选同类产品更多 >
万千商家帮你免费找货
0 人在求购买到急需产品
- 详细信息
- 用户评价
- 文献和实验
- 技术资料
- 库存:
请询
- 英文名:
SafetyTector™
- CAS号:
N/A
- 保质期:
please refer to the label on the bottle
- 供应商:
青木生物技术(武汉)有限公司
- 保存条件:
2-8 °C
- 规格:
1L
SafetyTector™ S is a ready-to-use diluent for immunoassays based on saliva or mucosal swab samples. Diluted samples are directly applied to lateral flow assays as flow buffer. Swabs can be extracted directly with SafetyTector™ S. Diluted samples can also be used in other immunoassays such as ELISA. SafetyTector™ S inactivates SARS-CoV-2 and improves the texture of potentially infectious saliva or mucosal swab samples. At a 1:4 dilution, SafetyTector™ S is able to inactivate SARS-CoV-2 in these samples within 1 min1 (For details please refer to the „Letter of Acknowledgement“issued by the Institute of Molecular Virology, University Hospital Ulm).
Instructions for use
SafetyTector™ S is ready-to-use. Please shake the buffer thoroughly before use.
Dilution of the specimens: Samples must be diluted in SafetyTector™ S at least 1:4 (1 part sample in 3 parts SafetyTector™ S) and mixed thoroughly. Standards and specimens should be treated strictly the same way. SafetyTector™ S is not suited for long-term storage of specimens.
Lateral flow assays: SafetyTector™ S replaces the dilution buffer, extraction buffer, chase buffer or flow buffer. Saliva must be diluted with at least 3 sample volumes of SafetyTector™ S. Swabs must be incubated in SafetyTector™ S and should be squeezed for optimal sample recovery, if applicable. Volume of SafetyTector™ S must at least be 3 times the maximum capacity of the swab.
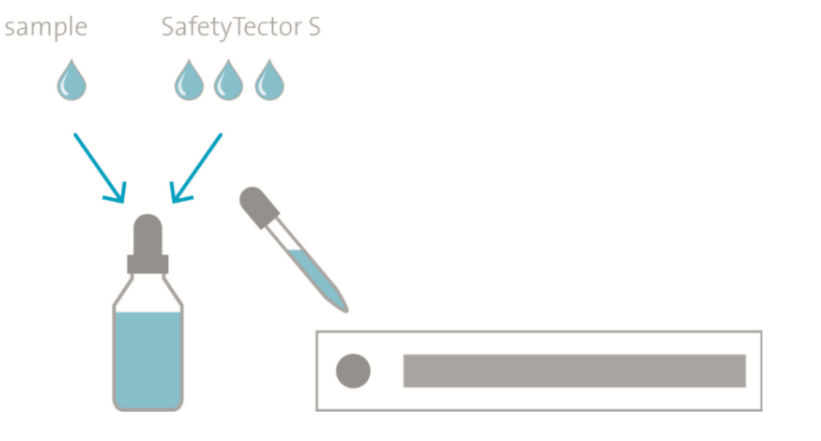
Other assay technologies: SafetyTector™ S may also be employed as sample diluent if saliva or mucosal swab samples are analysed by technologies like ELISA, protein arrays, bead assays (e.g. Luminex assays), immuno-PCR, or automated high-throughput immunoassay systems.
Suitability of SafetyTector™ S for a specific assay has to be tested by the user.
For further information please visit www.candor-bioscience.com
文献引用:
- I. White et al. (2021). Bifunctional molecules targeting SARS-CoV-2 spike and the polymeric Ig receptor display neutralization activity and mucosal enrichment. In mAbs (Vol. 13, No. 1, p. 1987180). Taylor & Francis. Y. Liu et al. (2021).
- Paradoxical Sex-Specific Patterns of Autoantibodies Response to SARS-CoV-2 Infection. Journal of Translational Medicine, 19(1), 1-13. A. Athanasiou et al. (2021).
- A novel serum biomarker quintet reveals added prognostic value when combined with standard clinical parameters in prostate cancer patients by predicting biochemical recurrence and adverse pathology. Plos one, 16(11), e0259093. X. T. Zhang et al. (2021).
- A competitive ligand-binding assay for the detection of neutralizing antibodies against dostarlimab (TSR-042). AAPS Open, 7(1), 1-14. J. M. Chan et al. (2021).
- Signatures of plasticity, metastasis, and immunosuppression in an atlas of human small cell lung cancer. Cancer Cell, 39(11), 1479-1496. F. Rüger et al. (2021).
- Gold albumin Sandwich Structures for Enhanced Biosensing using Surface Plasmon Resonance. physica status solidi (a). doi.org/10.1002/pssa.202100029 L. He et al. (2021).
- The effects of CD148 Q276P/R326Q polymorphisms in A431D epidermoid cancer cell proliferation and epidermal growth factor receptor signaling. Cancer Reports, e1566. B. Henrick et al. (2021).
- Bifidobacteria-mediated immune system imprinting early in life. Cell 184(15), 3884-3898.e11.
风险提示:丁香通仅作为第三方平台,为商家信息发布提供平台空间。用户咨询产品时请注意保护个人信息及财产安全,合理判断,谨慎选购商品,商家和用户对交易行为负责。对于医疗器械类产品,请先查证核实企业经营资质和医疗器械产品注册证情况。
 用户评价
用户评价 暂无用户评价
暂无用户评价 文献和实验
文献和实验- I. White et al. (2021). Bifunctional molecules targeting SARS-CoV-2 spike and the polymeric Ig receptor display neutralization activity and mucosal enrichment. In mAbs (Vol. 13, No. 1, p. 1987180). Taylor & Francis. Y. Liu et al. (2021).
- Paradoxical Sex-Specific Patterns of Autoantibodies Response to SARS-CoV-2 Infection. Journal of Translational Medicine, 19(1), 1-13. A. Athanasiou et al. (2021).
- A novel serum biomarker quintet reveals added prognostic value when combined with standard clinical parameters in prostate cancer patients by predicting biochemical recurrence and adverse pathology. Plos one, 16(11), e0259093. X. T. Zhang et al. (2021).
- A competitive ligand-binding assay for the detection of neutralizing antibodies against dostarlimab (TSR-042). AAPS Open, 7(1), 1-14. J. M. Chan et al. (2021).
- Signatures of plasticity, metastasis, and immunosuppression in an atlas of human small cell lung cancer. Cancer Cell, 39(11), 1479-1496. F. Rüger et al. (2021).
- Gold albumin Sandwich Structures for Enhanced Biosensing using Surface Plasmon Resonance. physica status solidi (a). doi.org/10.1002/pssa.202100029 L. He et al. (2021).
- The effects of CD148 Q276P/R326Q polymorphisms in A431D epidermoid cancer cell proliferation and epidermal growth factor receptor signaling. Cancer Reports, e1566. B. Henrick et al. (2021).
- Bifidobacteria-mediated immune system imprinting early in life. Cell 184(15), 3884-3898.e11.
,病毒灭活缓冲液比保护措施(如手套、护目镜、防 撕收集袋)或安全信息(如产品说明书)更可取。 解决方案: SafetyTectorTMS 为了解决病毒灭活的问题,使所有市场参与者无需投入大量金钱和时间就能获得安全的稀释缓冲液,CANDOR开发出了SafetyTector™ S 。SafetyTector™ S 是一种即用型的病毒灭活稀释液和提取缓冲液,用于唾液样本和鼻咽拭子。同时,SafetyTector™ S 不是有害物质。此外,病毒灭活效果是在不使用对环境有害的灭活病毒成分的情况
进行标准化,预估扩增靶点的相对表达水平[1,2]。如今,终点 PCR 已基本被实时 PCR 或 qPCR 取代了,因为它们可获得更可靠和更准确的基因表达定量结果。 图 2:起始 cDNA 连续稀释液的 PCR 得率,通过在琼脂糖凝胶上对 PCR 产物染色进行可视化观察。 2.基因分型 PCR 可用于检测特定细胞或生物体中等位基因的序列差异。例如,基因敲除和敲入小鼠等转基因生物的基因分型[3]。引物对经设计位于目标区域侧翼,可根据是否存在扩增子及扩增子长度来检测遗传变异(图 3)。 图 3.PCR 用于
计数细胞 (PHCC360KIT),并用 3dGRO™ 球状体培养基调节其体积,为每个接种密度(表 1)准备 5 mL 稀释液,以便每个时间点的每个接种密度可接种 8 个孔(四个 96 孔球形微孔板)。 表1.接种密度制备 将 100 µL 细胞悬液分装至孔中,每个浓度的8 个孔。对照孔分别加入 100 μL 的无细胞3dGRO™ 球状体培养基。 注:球形微孔板可以使用多通道移液管手动接种*,或使用液体进样针自动接种**。对于 96 孔和 384 孔球形微孔板,仅需手动添加
 技术资料
技术资料需要更多技术资料 索取更多技术资料
资料下载:
safetytector-rapid-test-candor中文.pdf 附 (下载 0 次)
safetytector-package-insert.pdf 附 (下载 0 次)
请 [登录] 后再下载!