相关产品推荐更多 >
万千商家帮你免费找货
0 人在求购买到急需产品
- 详细信息
- 询价记录
- 文献和实验
- 技术资料
- 现货状态:
现货
- 批准文号:
见瓶身
- 库存:
大量
- 产品用途:
注射
- 曾用名:
DOPG
- 英文名:
Dioleoyl Phosphatidylglycerole
- 供应商:
艾伟拓(上海)医药科技有限公司
- 保质期:
24个月
- 执行标准:
进口药品注册标准
- 规格:
件/件
| 规格: | 件 | 产品价格: | ¥10.0 |
|---|---|---|---|
| 规格: | 件 | 产品价格: | ¥99.0 |
1、中文通用名:二油酰磷脂酰甘油
2、中文化学名:L-α-磷脂酰-DL-甘油,二油酰,钠盐
3、英文名:DOPG
4、英文化学名:L-α-Phosphatidyl-DL-glycerol,dioleyl,sodium salt
5、CAS号:62700-69-0
6、分子量:797.04
7、分子式:C42H78O10PNa
8、级别:试剂
9、保存方式: -20℃以下,遮光密闭
10、产品特性:白色颗粒,不溶于水
11、结构式: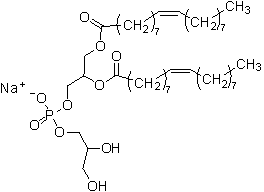

日本精化:
日本精化株式会社,作为综合性精密化学品制造厂商的全新开始,现在事业领域的成立以化妆品香料、工业用化学品、以及医药品为首的精密化学品领域的三根梁柱。
在面向全球化进展,企业间的竞争力更为激烈的当今,本公司在推进集中与选择的同时,也在向成为国际化、国际成本竞争力的获得而日日迈进。
本公司的特征是灵活运用本公司独创技术,以樟脑,脂肪酸,甜性,磷脂类体等对地球无公害的天然原料做为主要事业的主原料,为客户稳定提供高品质的产品,我们自信于我们的产品在日本国内乃至国际上都得到了很高的评价。
关于AVT
艾伟拓(上海)医药科技有限公司成立于2007年,服务中国制剂产业,致力为高端制剂提供特种辅料和高品质服务。 艾伟拓精准定位注射用辅料,六大产品线:核酸递送类全套辅料;生物制剂辅料;脂质体用合成磷脂;脂肪乳用天然磷脂;医美用透明质酸等高分子材料;新型疫苗佐剂,已覆盖3800+终端客户、1000+药厂客户、1700+科研机构、16+高校采购平台。


Founded in 2007, AVT (Shanghai) Pharmaceutical Tech Co., Ltd. (hereinafter referred to as AVT) specializes in providing high quality excipients and services for advanced injections.
The company’s products range from high quality traditional injection grade phospholipid excipients applied for liposomes and fat emulsions, such as natural phospholipids, HSPC, DSPE-PEG2000, CHO, etc.; as well as protective agents and buffers salts used in vaccines, antibody proteins, antibody-conjugated drugs and other biological formulations, such as trehalose, sucrose, Tris Base,HEPES, etc.
AVT has always pursued to develop high purity, low endotoxin excipients aimed for injections. Our products are manufactured in accordance with GMP guidelines and under strict in-house quality control standards, these products can meet requirements of mainstream pharmacopoeias including ChP, NF, EP, and JP. We have established steady cooperation with domestic mainstream pharmaceutical companies and emerging biological companies.

风险提示:丁香通仅作为第三方平台,为商家信息发布提供平台空间。用户咨询产品时请注意保护个人信息及财产安全,合理判断,谨慎选购商品,商家和用户对交易行为负责。对于医疗器械类产品,请先查证核实企业经营资质和医疗器械产品注册证情况。
- 作者
- 内容
- 询问日期
 文献和实验
文献和实验[1] CHEN G, KANG W, LI W, et al. Oral delivery of protein and peptide drugs: from non-specific formulation approaches to intestinal cell targeting strategies [J]. Theranostics, 2022, 12(3): 1419-1439.
[2] PARK K, KWON I C, PARK K. Oral protein delivery: current status and future prospect [J]. React Funct Polym, 2011, 71(3): 280-287.
[3] LUNDQUIST P , ARTURSSON P . Oral absorption of peptides and nanoparticles across the human intestine: opportunities, limitations and studies in human tissues [J]. Adv Drug Deliv Rev, 2016, 106(Part B): 256-276.
[4] TONG T, W ANG L, YOU X, et al. Nano and microscale delivery platforms for enhanced oral peptide/protein bioavailability [J]. Biomater Sci, 2020, 8(21): 5804-5823.
[5] SILV A A C, SANTOS D, FERREIRA D, et al. Lipid-based nanocarriers as an alternative for oral delivery of poorly water- soluble drugs: peroral and mucosal routes [J]. Curr Med Chem, 2012, 19(26): 4495-4510.
[6] SARMENTO B, MAZZAGLIA D, BONFERONI M C, et al. Effect of chitosan coating in overcoming the phagocytosis of insulin loaded solid lipid nanoparticles by mononuclear phagocyte system [J]. Carbohyd Polymers, 2011, 84(3): 919-925.
[7] HADDADZADEGAN S, DORKOOSH F, BERNKOP-SCHNÜRCH A. Oral delivery of therapeutic peptides and proteins: technology landscape of lipid-based
nanocarriers [J]. Adv Drug Deliv Rev, 2022, 182: 114097.
[8] SHAN W, ZHU X, LIU M, et al. Overcoming the diffusion barrier of mucus and
absorption barrier of epithelium by self-assembled nanoparticles for oral delivery of insulin [J]. ACS Nano, 2015, 9(3): 2345-2356.
[9] ZUPANČIČ O, BERNKOP-SCHNÜRCH A. Lipophilic peptide character - what
oral barriers fear the most [J]. J Control Release, 2017, 255: 242-257.
[10] RISTROPH K D, PRUD'HOMME R K. Hydrophobic ion pairing: encapsulating small molecules, peptides, and proteins into nanocarriers [J]. Nanoscale Adv, 2019, 1(11): 4207-4237.
[11] MUNTONI E, MARINI E, AHMADI N, et al. Lipid nanoparticles as vehicles for oral delivery of insulin and insulin analogs: preliminary ex vivo and in vivo studies [J]. Acta Diabetol, 2019, 56(12): 1283-1292.
[12] SMART A L, GAISFORD S, BASIT A W. Oral peptide and protein delivery: intestinal obstacles and commercial prospects [J]. Expert Opin Drug Deliv, 2014, 11(8): 1323-1335.
[13] ARNOLD Y E, IMANIDIS G, KUENTZ M. In vitro digestion kinetics of
excipients for lipid-based drug delivery and introduction of a relative lipolysis half life [J]. Drug Dev Ind Pharm, 2012, 38(10): 1262-1269.
[14] DAMGÉ C, REIS C P , MAINCENT P . Nanoparticle strategies for the oral delivery
of insulin [J]. Expert Opin Drug Deliv, 2008, 5(1): 45-68.
[15] BA TTAGLIA L, GALLARA TE M. Lipid nanoparticles: state of the art, new preparation methods and challenges in drug delivery [J]. Expert Opin Drug Deliv, 2012, 9(5): 497-508.
[16] DAS S, CHAUDHURY A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery [J]. AAPS PharmSciTech, 2011, 12(1): 62-76.
[17] MEANEY C M, O'DRISCOLL C M. A comparison of the permeation
enhancement potential of simple bile salt and mixed bile salt: fatty acid micellar systems using the CaCo-2 cell culture model [J]. Int J Pharm, 2000, 207(1/2): 21-30.
[18] ZHANG Z, GAO F, JIANG S, et al. Bile salts enhance the intestinal absorption of lipophilic drug loaded lipid nanocarriers: mechanism and effect in rats [J]. Int J Pharm, 2013, 452(1/2): 374-381.
[19] MCCLEMENTS D J. Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: a review [J]. Adv Colloid Interface Sci, 2018, 253: 1-22.
[20] MUCHOW M, MAINCENT P , MULLER R H. Lipid nanoparticles with a solid matrix (SLN, NLC, LDC) for oral drug delivery [J]. Drug Dev Ind Pharm, 2008, 34(12): 1394-1405.
[21] SAINI A, PANW AR D, PANESAR P S, et al. Encapsulation of functional ingredients in lipidic nanocarriers and antimicrobial applications: a review [J]. Environ Chem Lett, 2021, 19: 1107-1134.
[22] BANERJEE S, PILLAI J. Solid lipid matrix mediated nanoarchitectonics for improved oral bioavailability of drugs [J]. Expert Opin Drug Metab Toxicol, 2019, 15(6): 499-515.
B. Maruo和 A. A. Benson( 1958)在栅藻属( Scenedesmus)细胞的醇抽提物中发现的磷脂的主要成分。广泛分布于生物界,在微生物中,有时也是磷脂的主要成分。与心磷脂,磷脂酰肌醇一样,是一种酸性磷脂。在生长中的大肠杆菌中,它的代谢速率较其它磷脂为高。它是由 CDP甘油酯与磷酸甘油生物合成为磷酸磷脂酰甘油,再通过脱磷酸而形成为磷脂酰甘油。天然的磷脂酰甘油是二酰基 -L- 3-磷酸甘油 -D- 3-甘油。通过磷脂
心磷脂 cardiolipin 一种磷脂,亦称双磷脂酰甘油( diphosphatid- ylglycerol)。是潘伯恩( M. C. Pangborn) 1941年从新鲜的牛心肌中分离出来的,显示有梅毒患者血清诊断的特异性抗原。广泛存在于高等动物、植物、微生物界。在动物细胞中主要存在于线粒体的内膜,特别在心肌甚至占总磷脂的 15%,在牛心肌的心磷脂中脂肪酸(结构式中以 R, R′, R″, R″表示)残基的 80— 90%是亚油酸。把心磷脂与卵磷脂及胆甾醇以一定的比例
酸和碱基,广泛存在于菠菜、甜菜、甘兰叶、胡罗卜等植物中。酶活性局限于质体及叶绿体中,最适 pH为 5.0— 6.0,最易分解卵磷酯。此酶也具有转移磷脂酰基的活性,在卵磷酯和甘油的存在下起反应,生成磷脂酸和磷脂酰甘油。此外,在副流感嗜血菌中( Haemophilus parainfluenzae)还发现特异分解心磷酯( cardiolipin)的磷脂酶 D。











