相关产品推荐更多 >
万千商家帮你免费找货
0 人在求购买到急需产品
- 详细信息
- 文献和实验
- 技术资料
- 英文名:
K7M2-WT
- 库存:
100万
- 供应商:
欣润生物
- 肿瘤类型:
骨肉瘤
- 细胞类型:
细胞系
- ATCC Number:
详见说明
- 品系:
Balb/c
- 组织来源:
骨肉瘤
- 相关疾病:
骨肉瘤
- 物种来源:
小鼠
- 免疫类型:
不详
- 细胞形态:
成骨细胞样
- 是否是肿瘤细胞:
是
- 器官来源:
骨
- 运输方式:
新鲜或干冰
- 年限:
成年
- 生长状态:
贴壁生长
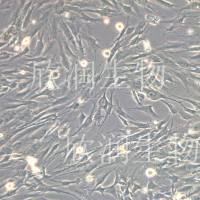
- 细胞名称:K7M2-WT细胞(小鼠骨肉瘤成骨细胞)
- 形态:成骨细胞样,贴壁生长
- 含量:>1x106 个/瓶
- 污染:支原体、细菌、酵母和真菌检测为阴性
- 规格:T25瓶或者1mL冻存管包装
二、细胞接收后的处理:
1、贴壁细胞
- 收到T25方瓶细胞后,请检查是否漏液,如果漏液,请拍照片发给我们(冻存管细胞收到后直接37℃水浴复苏或直接放置于液氮中长期储存)。
- 请先在显微镜下确认细胞生长状态,去掉封口膜并将T25瓶置于37℃培养约2-3h。
- 弃去T25瓶中的培养基,换用新鲜的完全培养基。
- 如果细胞长满(90%以上)请及时进行细胞传代。
- 接到细胞次日,请检查细胞是否污染,若发现污染或疑似污染,请及时与我们取得联系。
2、悬浮细胞
- 收到细胞后,请检查是否漏液,如果漏液,请拍照片发给我们。
- 请先在显微镜下确认细胞生长状态,去掉封口膜并将15ml离心管置于37℃培养约2-3h。
- 1200rpm离心5min,弃去15ml离心管中的培养基,细胞沉淀用新鲜的完全培养基重悬并培养。
- 如果细胞长满(90%以上)请及时进行细胞传代。
- 接到细胞次日,请检查细胞是否污染,若发现污染或疑似污染,请及时与我们取得联系。
本公司的细胞培养操作规程,供参考
一、培养基及培养冻存条件准备:
- 准备H-DMEM培养基,90%;优质胎牛血清,10%。
- 培养条件: 气相:空气,95%;二氧化碳,5%。 温度:37℃,培养箱湿度为70%-80%。
- 冻存液:90%血清,10%DMSO,现用现配。液氮储存。
对于贴壁细胞,传代可参考以下方法:
- 弃去培养上清,用不含钙、镁离子的PBS润洗细胞1-2次。
- 加2ml消化液(0.25%Trypsin-0.53mM EDTA)于培养瓶中,置于37℃培养箱中消化2-3分钟,然后在显微镜下观察细胞消化情况,若细胞大部分变圆并脱落,迅速拿回操作台,轻敲几下培养瓶后加入3ml此细胞的培养基终止消化。
- 轻轻吹打后吸出,移入15ml离心管中,在1200RPM条件下离心5分钟,弃去上清液,加入1mL培养液后吹匀。
- 移入到事先准备好的含有5ml培养基的T-25培养瓶中或含有14ml培养基的T-75培养瓶中培养。
3)细胞冻存:待细胞生长状态良好时,可进行细胞冻存。贴壁细胞冻存时,先要消化处理并进行细胞计数。消化方法按照细胞传代方法的1-3步骤进行,最后的重悬液使用血清。悬浮细胞直接计数后离心,用血清重悬浮,加DMSO至最终浓度为10%。加入DMSO后迅速混匀,按每1ml的数量分配到冻存管中。本公司按每个冻存管细胞数目大于1X106个细胞冻存。
注意事项:
1. 收到冻存管细胞后,若发现干冰已挥发干净、冻存管瓶盖脱落、破损及细胞有污染,请立即与我们联系。
2. 所有动物细胞均视为有潜在的生物危害性,必须在二级生物安全台内操作,并请注意防护,所有废液及接触过此细胞的器皿需要灭菌后方能丢弃。
3. 细胞用途:仅供科研使用。
发货方式:
复苏后发货:我们复苏细胞后发货,货期一周左右,免运费。(气温较好建议复苏后发货)
冻存发货(干冰运输):需额外增加干冰运费,选择干冰运输的我们发两管细胞,为了保证客户接种可靠性多发一管。(气温低于0℃须冻存发货)
细胞发货采取专业的运输包装,并选择最快捷的运输方式(顺丰速运或其他空运快递)
Highly Metastatic K7M2 Cell Line: A Novel Murine Model Capable of In Vivo Imaging via Luciferase Vector Transfection
Osteosarcoma is rare and little improvement in survival rates has occurred in the last 25 years despite modern chemotherapeutic treatment. Bioluminescent cell lines for the modeling of osteosarcoma have shown success in tracking metastases in vivo , but commonly use adenoviral vectors to transfect the native cell line with bioluminescent reporters. The purpose of this study was to develop an orthotopic model for metastatic osteosarcoma capable of in vivo monitoring of metastatic and primary tumor burden in an immunocompetent mouse and compare that model to its wild type pathogenesis. K7M2 cells were transfected using a plasmid vector and were stable after 12 weeks. Thirty-four female BALB/c mice aged four to five weeks underwent orthotopic implantation of either wild type ( n = 12 ) or transfected ( n = 22 ) K7M2 cells in the proximal tibia. Mice were monitored for tumor growth and weekly In Vivo Imaging System ( IVIS ) imaging was performed to monitor for pulmonary metastasis. Although tumors developed sooner in the wild type group, no significant differences were seen compared to Transfected Group 1 in rate of inoculation, growth rates after first detection, metastatic rate, and time between inoculation and death. This study establishes a new murine model for metastatic osteosarcoma using the K7M2-wt cell line transfected with a non-viral plasmid luciferase vector. The benefits of this preclinical model include an intact immune system and orthotopically driven metastatic disease; this model appears comparable to its wild type counterpart. In the future, the model may be used to examine promising immunomodulatory therapies using bioluminescence in vivo .
PTHR1 May Be Involved in Progression of Osteosarcoma by Regulating miR-124-3p-AR-Tgfb1i1, miR-27a-3p-PPARG-Abca1, and miR-103/590-3p-AXIN2 Axes
Our previous study has indicated that the parathyroid hormone type 1 receptor (PTHR1) may play important roles in development and progression of osteosarcoma (OS) by regulating Wnt, angiogenesis, and inflammation pathway genes. The goal of this study was to further illuminate the roles of PTHR1 in OS by investigating upstream regulation mechanisms (including microRNA [miRNA] and transcription factors [TFs]) of crucial genes. The microarray dataset GSE46861 was downloaded from the Gene Expression Omnibus database, in which six tumors with short hairpin RNA (shRNA) PTHR1 knockdown (PTHR1.358) and six tumors with shRNA control knockdown (Ren.1309) were collected from mice. Differentially expressed genes (DEGs) between PTHR1.358 and Ren.1309 were identified using the linear models for microarray data (LIMMA) method, and then the miRNA-TF-mRNA regulatory network was constructed using data from corresponding databases, followed by module analysis, to screen crucial regulatory relationships. OS-related human miRNAs were extracted from the curated Osteosarcoma Database. Gene ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were enriched using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) tool. As a result, the miRNA-TF-mRNA regulatory network, including 1049 nodes (516 miRNA, 25 TFs, and 508 DEGs) and 15942 edges (interaction relationships, such as Pparg-Abca1 and miR-590-3p-AXIN2), was constructed, from which three significant modules were extracted and modules 2 and 3 contained interactions between miRNAs/TFs and DEGs such as miR-103-3p-AXIN2, miR-124-3p-AR-Tgfb1i1, and miR-27a-3p-PPARG-Abca1. miR-27a-3p was a known miRNA associated with OS. Abca1, AR, and miR-124-3p were hub genes in the miRNA-TF-mRNA network. Tgfb1i1 was involved in cell proliferation, Abca1 participated in the cholesterol metabolic process, and AXIN2 was associated with the canonical Wnt signaling pathway. Furthermore, we also confirmed upregulation of miR-590-3p and downregulation of AXIN2 in the mouse OS cell line K7M2-WT transfected with PTHR1 shRNA. In conclusion, PTHR1 may play important roles in progression of OS by activating miR-124-3p-AR-Tgfb1i1, miR-27a-3p-PPARG-Abca1, and miR-103/590-3p-AXIN2 axes.
风险提示:丁香通仅作为第三方平台,为商家信息发布提供平台空间。用户咨询产品时请注意保护个人信息及财产安全,合理判断,谨慎选购商品,商家和用户对交易行为负责。对于医疗器械类产品,请先查证核实企业经营资质和医疗器械产品注册证情况。
 文献和实验
文献和实验Chicken intestinal epithelial cells were obtained from NEWGAINBIO company. Cells were cultured on 37℃, with 5% CO2, in the Ham’s F-12 Nutrient (DMEM/12) that contained the following supplementations: fetal bovine serum (5%), in-sulin (5 µg/mL), transferrin (5 µg/mL), selenium (5 ng/mL), epidermal growth factor (5 ng/mL) and penicillin-streptomycin (100–100 U/mL) for cell culturing (full DMEM/12). Experiments were performed with chicken intestinal epithelial cells and working solutions were prepared with plain DMEM/12 without supplementation. For the investigations, cells were seeded onto 96-well, 24-well or 6-well polystyrene cell culture plates.
Primary hVICs (passage 2) were cultured to 50–60% confluence and infected with pGMLV-SV40T-puro lentivirus (NewgainBio, Wuxi, China) at a multiplicity of infection of 80 supplemented with 5 µg/mL polybrene (Sigma-Aldrich, Buchs, Switzerland).
Tissue was cultured until cells became visible around the tissue, and when the fusion reached 90% (FIGURE 1A) §ask ¦lled with the prepared culturing medium was sent to the company for further immortalisation. Cell immortalisation was done for cell stability and longer-term use. Immortalised cells were cultured with 10% FBS and 1% PS in the DMEM medium. After the cells multiplied and merged, they were routinely passed and grown ( NEWGAINBIO Inc. Wuxi, Jiangsu, China) (FIGURE 1B-C).
Mouse primary cultured renal vascular ECs and VSMCs were obtained from Newgainbio company, which were tested by Factor VIII and α-smooth muscle actin (α-SMA), the marker of ECs and VSMCs. RNeasy Mini Kit was used for RNA extraction, and the above protocols were repeated.
Porcine primary colon epithelial cells (Newgainbio company, Wuxi,China) were cultured in Dulbecco's Modified Eagle's Medium (Solarbio, Beijing, China) containing 10 % fetal bovine serum (BioInd, Kiryat shmona, Lsrael) at 37 ◦C and 5 % CO2 humidity.