万千商家帮你免费找货
0 人在求购买到急需产品
- 详细信息
- 询价记录
- 文献和实验
- 技术资料
- 保存条件:
-20ºC
- 保质期:
一年
- 英文名:
HSP70 Protein
- 库存:
大量
- 供应商:
StressMarq
- 规格:
50 µg/100 µg/100 µg x2
| 规格: | 50 µg | 产品价格: | 询价 |
|---|---|---|---|
| 规格: | 100 µg | 产品价格: | 询价 |
| 规格: | 100 µg x2 | 产品价格: | 询价 |
| 产品名称 | HSP70 蛋白 |
| 产品描述 |
活性重组人HSP70全长蛋白 |
| 应用范围 | WB, SDS-PAGE, ATPase Activity Assay, Functional Assay, ELISA |
| 浓度 | 各批次不同,请详见说明书 |
| 标记物 | 无标签 |
| 性质 | 重组 |
| 来源物种 | 人 |
| 表达系统 | 大肠杆菌 (E. coli) |
| 氨基酸序列 | MAKAAAIGIDLGTTYSCVGVFQHGKVEIIANDQGNRTTPSYVAFTDTERLIGDAAKNQVALNPQNTVFDAKRLIGRKFGDPVVQSDMKHWPFQVINDGDKPKVQVSYKGETKAFYPEEISSMVLTKMKEIAEAYLGYPVTNAVITVPAYFNDSQRQATKDAGVIAGLNVLRIINEPTAAAIAYGLDRTGKGERNVLIFDLGGGTFDVSILTIDDGIFEVKATAGDTHLGGEDFDNRLVNHFVEEFKRKHKKDISQNKRAVRRLRTACERAKRTLSSSTQASLEIDSLFEGIDFYTSITRARFEELCSDLFRSTLEPVEKALRDAKLDKAQIHDLVLVGGSTRIPKVQKLLQDFFNGRDLNKSINPDEAVAYGAAVQAAILMGDKSENVQDLLLLDVAPLSLGLETAGGVMTALIKRNSTIPTKQTQIFTTYSDNQPGVLIQVYEGERAMTKDNNLLGRFELSGIPPAPRGVPQIEVTFDIDANGILNVTATDKSTGKANKITITNDKGRLSKEEIERMVQEAEKYKAEDEVQRERVSAKNALESYAFNMKSAVEDEGLKGKISEADKKKVLDKCQEVISWLDANTLAEKDEFEHKRKELEQVCNPIISGLYQGAGGPGPGGFGAQGPKGGSGSGPTIEEVD |
| 纯度 | >90% |
| 蛋白长度 | 全长蛋白 |
| Full Biological Activity | ATPase 活性 |
产品特性
| 储存缓冲液 | 50mM Tris/HCl pH7.5, 2.5mM Bme, 0.15M NaCl, 10% 甘油 |
| 储存温度 | -20ºC |
| 运输温度 | 蓝冰或4℃ |
| 纯化方式 | 多步骤色谱法 |
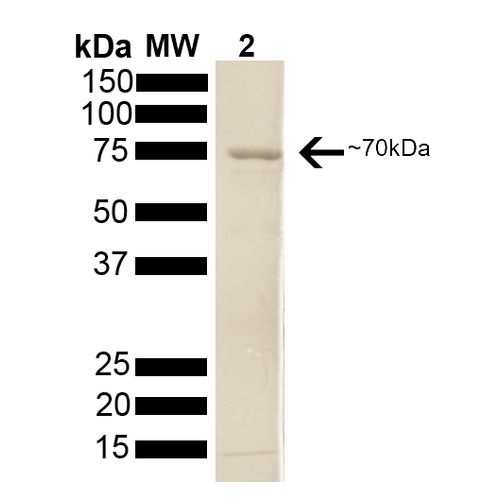
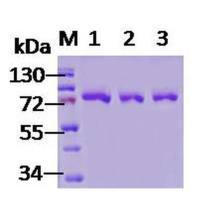
| Protein Size | 分子量约为70kD |
| 引用该产品 | Human Recombinant HSP70 Protein (StressMarq Biosciences Inc., Victoria BC CANADA, Catalog # SPR-108) |
| 分析证书 | 该蛋白已经通过SDS-PAGE检测证明纯度大于90%. 此蛋白出厂时的ATP酶活性为每小时从每微克蛋白质分离出3.0μM 磷酸根 (使用孔雀绿磷酸检测, 200μl反应液, 20ul的1mM ATP, 37°C, pH7.5). |
| 其他相关信息 | 具有ATP酶活性, 原蛋白序列 |
生物学特性
| 别名 | Hsp70 1 Protein, Hsp70 2 Protein, Hsp70.1 Protein, Hsp72 Protein, Hsp73 Protein, HSPA1 Protein, HSPA1A Protein, HSPA1B Protein |
| 研究领域 | 伴侣蛋白, 热休克, 癌症, 细胞信号传导, 蛋白质运输, 肿瘤标记物 |
| 细胞定位 | 细胞质 |
| Accession Number | NM_005345 |
| GeneID | 3303 |
| Swiss Prot | P0DMV8/P0DMV9 |
| 科研背景 | HSP70 genes encode abundant heat-inducible 70-kDa HSPs (HSP70s). In most eukaryotes HSP70 genes exist as part of a multigene family. They are found in most cellular compartments of eukaryotes including nuclei, mitochondria, chloroplasts, the endoplasmic reticulum and the cytosol, as well as in bacteria. The genes show a high degree of conservation, having at least 5O% identity (2). The N-terminal two thirds of HSP70s are more conserved than the C-terminal third. HSP70 binds ATP with high affinity and possesses a weak ATPase activity which can be stimulated by binding to unfolded proteins and synthetic peptides (3). When HSC70 (constitutively expressed) present in mammalian cells was truncated, ATP binding activity was found to reside in an N-terminal fragment of 44kDa which lacked peptide binding capacity. Polypeptide binding ability therefore resided within the C-terminal half (4). The structure of this ATP binding domain displays multiple features of nucleotide binding proteins (5). All HSP70s, regardless of location, bind proteins, particularly unfolded ones. The molecular chaperones of the HSP70 family recognize and bind to nascent polypeptide chains as well as partially folded intermediates of proteins preventing their aggregation and misfolding. The binding of ATP triggers a critical conformational change leading to the release of the bound substrate protein (6). The universal ability of HSP70s to undergo cycles of binding to and release from hydrophobic stretches of partially unfolded proteins determines their role in a great variety of vital intracellular functions such as protein synthesis, protein folding and oligomerization and protein transport. Looking for more information on HSP70? Visit our new HSP70 Scientific Resource Guide. |
| 参考资料 | 1. Zho J. (1998) Cell. 94: 471-480. 2. Boorstein, W. R., Ziegelhoffer, T. & Craig, E. A. (1993) J. Mol. Evol. 38(1): 1-17. 3. Rothman J. (1989) Cell. 59: 591 -601. 4. DeLuca-Flaherty et al. (1990) Cell. 62: 875-887. 5. Bork P., Sander C. & Valencia A. (1992) Proc. Natl Acad. Sci. USA. 89: 7290-7294. 6. Fink A.L. (1999) Physiol. Rev. 79: 425-449. 7. Smith D.F., et al., (1993) Mol. Cell. Biol. 13(2): 869-876. 8. Prapapanich V., et al., (1996) Mol. Cell. Biol. 16(11): 6200-6207. 9. Fernandez-Funez et al., (2000) Nature. 408(6808): 101-106. |
产品图片
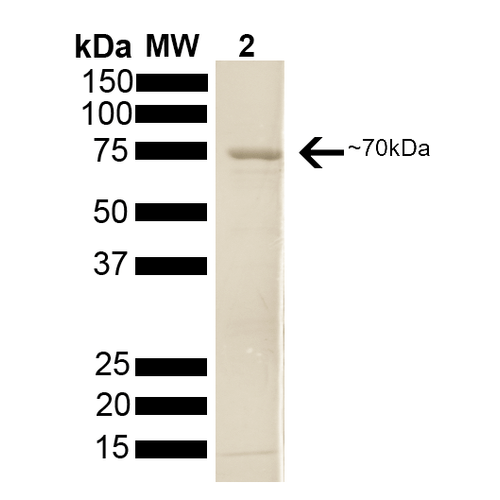
70kDa Hsp70 蛋白 SDS-PAGE (SPR-108).
风险提示:丁香通仅作为第三方平台,为商家信息发布提供平台空间。用户咨询产品时请注意保护个人信息及财产安全,合理判断,谨慎选购商品,商家和用户对交易行为负责。对于医疗器械类产品,请先查证核实企业经营资质和医疗器械产品注册证情况。
- 作者
- 内容
- 询问日期
 文献和实验
文献和实验[1] Fong J J, Sreedhara K, Deng L, et al. Immunomodulatory activity of extracellular Hsp70 mediated via paired receptors Siglec‐5 and Siglec‐14[J]. The EMBO journal, 2015, 34(22): 2775-2788.
植物源重组人胰岛素生长因子融合蛋白(OsrIGF-1),胰岛素在无血清细胞培养中的最佳替代品。 IGFs家族由两种低分子多肽(IGF-Ⅰ、IGF-Ⅱ)、两类特异性受体及六种结合蛋白组成。IGF-Ⅰ是一个有70个氨基酸的单链碱性蛋白,分子量7649Da,耐热。IGFs的生物学功能不只局限于有丝分裂刺激作用,它们也能诱导分化或促进分化功能的表达。全新推出的植物源重组人类胰岛素生长因子-1融合蛋白(OsrIGF-1)在IGF-1的N末端融合了人血清白蛋白(HSA),HSA作为蛋白质保护剂增加了IGF
「生于忧患,死于安乐」,连细胞也是如此?剑桥大学研究揭示「压力」对细胞有何好处…
三分之一的蛋白的重任,还在重压之下促使蛋白质正确折叠!即对细胞施加压力,促使内质网细胞器发挥活性,并非通过降解或清除它们,而是通过拆开聚集体,使其正确地折叠! 二、「压力」触发热休克蛋白(HSP)的更高活性 对细胞施加压力,可促进聚集的蛋白质拆解并正确的折叠,那么如何唤醒这种机制呢? 深入的研究发现,该机制的主要成分是一种被称为热休克蛋白(HSP)的蛋白质,当细胞暴露于高于其正常生长温度的温度时,这类热休克蛋白会驱使内质网对压力作出反应。热休克蛋白 HSP70 及其辅助系统有助
Nature Communications|压力对细胞有何好处?
是带给人极大惊喜,不仅承担了生产体内三分之一的蛋白的重任,还在重压之下促使蛋白质正确折叠!即对细胞施加压力,促使内质网细胞器发挥活性,并非通过降解或清除它们,而是通过拆开聚集体,使其正确地折叠! 二、「压力」触发热休克蛋白(HSP)的更高活性 对细胞施加压力,可促进聚集的蛋白质拆解并正确的折叠,那么如何唤醒这种机制呢?深入的研究发现,该机制的主要成分是一种被称为热休克蛋白(HSP)的蛋白质,当细胞暴露于高于其正常生长温度的温度时,这类热休克蛋白会驱使内质网对压力作出反应。热休克蛋白 HSP70
 技术资料
技术资料暂无技术资料 索取技术资料