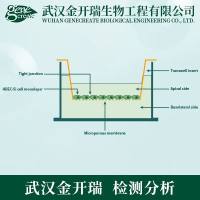
TransWell Chemotaxis
互联网
2518
| This protocol describes a method for studying chemotaxis (migration towards a concentration gradient of chemoattractant) of leukocytes (neutrophils, monocytes and lymphocytes) or other migratory cells. An upper chamber containing a suspension of cells is separated by a membrane from a lower chamber containing medium with chemoattractant. Chemotaxis of the cells from the upper chamber into the lower chamber occurs over a predetermined period of time. The cells in the bottom chamber may be counted directly (when using a homogeneous population of cells) or may be stained for defined markers, when assaying a mixed population of cells, and analyzed by flow cytometry. (See Protocol ID#2159 ) | ||||||||||||||||||||
|
Procedure |
||||||||||||||||||||
|
1. Prepare C-Buffer one day before the experiment to allow equilibration overnight in the incubator (see Hint #1 ). 2. Remove the 6.5 mm Transwell® filter inserts (Corning Costar Corporation) from the original 24-well plate (see Hint #2 ) and store in another 24-well plate. 3. Coat the wells of the original 24-well plate with Sigmacote (see Hint #3 ) and allow them to dry completely in the hood before proceeding. 4. Prepare appropriate dilutions of chemokines or other chemoattractants in C-Buffer (see Hint #4 ). Add 600 μl of a chemokine dilution to each well and place the plate in the CO2 incubator to equilibrate. 5. Prepare a suspension of the cells to be assayed and count them (see Protocol ID#2159 and Protocol ID#1934 ). 6. Pellet the cells by centrifugation (1000 x g) and resuspend in 37°C equilibrated C-Buffer at a concentration of 1 X 106 cells / 100 μl (see Hint #5 ). 7. Return the Transwell® filter inserts from Step #2 to the wells containing the chemoattractant dilutions. Add 100 μl of the cell suspension per well and return the plate to the incubator. 8. Incubate for the desired time (see Hint #6 ) and then remove from the incubator. Firmly tap the plate on the countertop to dislodge any loosely attached cells. 9. If monocytes and/or neutrophils are being assayed, add 50 μl of 70 mM EDTA to the lower well to release the cells that have adhered to the well and bottom of the membrane. Examine the wells under the microscope after 5 min to determine if the cells have detached. 10. Remove the filter inserts from the wells with forceps. Tap the insert against the edge of the well to remove any additional buffer. 11. Pipette 10,000 beads (15μm Dynospheres obtained from Bangs Laboratories) into each well (see Hint #7 ). Never add beads in less than 50 μl. Pre-rinse the tip several times before adding beads to the first well. Remix the beads after each row of wells (or more often if necessary) 13. Use a pipette to mix the contents of each well thoroughly. Transfer the contents to a FACS tube for staining or direct analysis. Count a sample on a FACScan, gating on both the cell population of interest and on the beads. Determine the total number of cells that migrated by dividing the number of counted cells by the number of counted beads and multiplying this number by 50,000 (the total number of beads in the sample). [(# of counted cells/# of counted beads) x 50,000] 14. Data can be expressed as a Migration Index: the number of cells that migrated in response to agonist relative to the number of cells that migrated randomly (i.e., to C-Buffer only). Alternatively, data can be expressed as a percentage of the starting cell population that migrated during the incubation period. For instance, if 106 cells were added to the upper chamber and 105 cells migrated to the bottom chamber, then 10% migration was achieved. Data can also be expressed as total cells migrated. |
||||||||||||||||||||
|
Solutions |
||||||||||||||||||||
|
||||||||||||||||||||
|
BioReagents and Chemicals |
||||||||||||||||||||
|
Sodium Chloride HBSS DMEM Sodium Bicarbonate Bovine Serum Albumin, endotoxin-free MgCl2 Potassium Phosphate, Monobasic HEPES DMEM, powder KCl MgSO4 Calcium Chloride EDTA Sodium Phosphate, Dibasic D-Glucose |
||||||||||||||||||||
|
Protocol Hints |
||||||||||||||||||||
|
1. An alternative method is to prepare the C-Buffer and infuse with CO2 prior to the experiment. 2. The optimal filter pore size varies, depending on the cell type used. Use filter inserts with 3 μm pores for neutrophils and monocytes; for lymphocytes, use 3 to 5 μm pores; for fibroblasts, use 8 μm pores. 3. Pipette 0.5 ml of Sigmacote into each well and aspirate immediately. Alternatively, pipette 0.5 ml of Sigmacote into the first well and then sequentially transfer this solution to the other wells in the plate. Aspirate the excess Sigmacote. 4. Chemokines are generally used in a concentration range from 0.01 nM to 100 nM. However, the optimal concentration range for a specific chemokine or chemotactic factor varies, and the literature should be consulted for the appropriate concentration range for a factor of interest. 5. Cells should be diluted immediately prior to addition to the Transwell® chamber. 6. Incubation times will depend on the cell type used. Incubate neutrophils for approximately 30 minutes. Incubate lymphocytes and monocytes for 3 to 4 hours. 7. The ratio of cells to beads will be used to calculate the total number of cells in each well. Therefore, the number of beads spiked in each well must be precise. Before the beads are added to the wells, they should be diluted in Chemotaxis Buffer to achieve a final concentration of 10,000 beads per 50 μl (never add beads in a volume less than 50 μl). Bead concentration is determined with a hemocytometer (see Protocol ID#1934 ). Do not use different pipettors for the addition of the beads. The beads should be mixed continuously during addition since they are very dense and do not stay in suspension very well. |