Nonradioactive Labeling and lmmunodetection of Recombinant Proteins
互联网
821
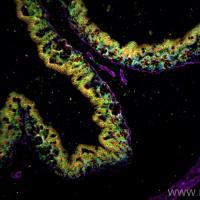
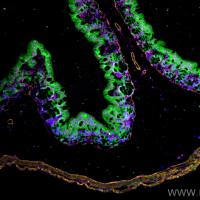
Recombinant proteins can be detected and characterized via several immunodetection schemes. Native proteins are typically detected by applying them to a membrane manually or using a vacuum manifold in a dot-or slot-blot format. This approach is suited to screening a number of samples for the presence of the recombinant protein of interest. When a more definitive characterization is desirable, Western blotting is performed, providing the apparent subunit molecular weight of the immunoreactive species. This IS accomplished by denaturing and reducing the sample, applying it to a sodium dodecyl sulfate (SDS) polyacrylamide gel, transferring the proteins to a suitable membrane, and carrying out immunodetection. Immunodetection of a dot, slot, or Western blot can be carried out using enzyme-labeled primary or secondary antibodybased systems or it can be performed using biotmylated primary or secondary antibodies with streptavidin or avidin-enzyme conlugates. Biotin is often the hapten of choice because of the extremely high dissociation constant for the biotin-avidin or biotin-streptavidin interaction. The dissociation constant is 10−15 /M (1 ). Erther streptavidin or avidin coqugates can be employed as biotin binding reagents but lower backgrounds are often observed with streptavidm-based detection schemes, so only streptavidin-based systems will be discussed.