Assay for a Galactosyltransferase Involved in the Assembly of the O7-Antigen Repeat Unit of Escherichia coli
互联网
互联网
相关产品推荐
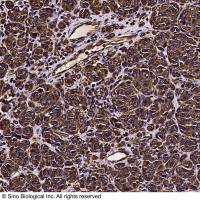
Vimentin Antibody, Rabbit PAb, Antigen Affinity Purified | Vimentin 兔多抗 (抗原亲和纯化)
¥1699

NAP1L4/NAP1L4蛋白/Nucleosome assembly protein 1-like 4(Nucleosome assembly protein 2)(NAP-2)蛋白/Recombinant Human Nucleosome assembly protein 1-like 4 (NAP1L4)重组蛋白
¥69

Recombinant-Saccharomyces-cerevisiae-3-hydroxyacyl-CoA-dehydratase-PHS1PHS13-hydroxyacyl-CoA dehydratase PHS1; HACD EC= 4.2.1.- Alternative name(s): PTPLA homolog involved in sphingolipid biosynthesis protein 1
¥10710

纤维素酶 酶混合物,9012-54-8,EnzymoPure™, ≥1000 unit/g,阿拉丁
¥720.90

Recombinant-Escherichia-coli-O7:K1-Zinc-transporter-ZupTzupTZinc transporter ZupT
¥11060
相关问答
相关方法

