Recycling Tubulin
互联网
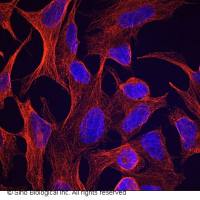
Recycling Tubulin
We "recycle" tubulin fractions stored at -80¡C after the PC column and store the recycled tubulin in small aliquots for day-to-day use. We generally store recycled tubulin in Injection Buffer (IB) without free GTP. We do this because depolymerization appears to be much better in IB, IB is ideal for microinjections/adding tubulin to extracts, and the absence of free GTP makes polymerization with GMPCPP, a very useful GTP analog that has ~5-10X lower affinity than GTP for tubulin, relatively straightforward.
-
I. Solutions & Supplies
-
II. Recycling Protocol
Back to protocols
I. Solutions & Supplies
5X BRB80 : (1X = 80 mM K-Pipes, 1 mM MgCl2 , 1 mM EGTA, pH 6.8 with KOH; store 5X BRB80 stock at 4¡C)
10X IB : 500 mM K-glutamate; 5 mM MgCl2 , (pH of 1X ~ 7.0; store 10X stock at -20¡C; see labeling section for details on making up IB)
Cushion : 60% glycerol in 1X BRB80 (warm = 37¡C)
100 mM GTP
Glycerol
Small Dounce (2 ml)
50.2Ti rotor (warm = 37¡C)
TLA100.2 or 100.3 rotor (cold = 2-4¡C)
II. Recycling Protocol
1. Thaw 3-4 3 ml PC column fractions at 37¡C. Transfer to ice and mix in a 50 ml conical.
2. Add BRB80 to 0.5X, 4 mM MgCl2 and 1 mM GTP. This is assuming that the tubulin aliquots are in CB (50 mM K-Pipes, pH 6.8; 1 mM EGTA; 0.2 mM MgCl2 ). Thus, the final buffer composition is 90 mM K-Pipes, pH 6.7, 1.5 mM EGTA, 4.2 mM MgCl2 ,1 mM GTP. Mix by swirling and store on ice for 5'.
3. Transfer to 37¡C. After 2' add half volume of glycerol (I find it convenient to pour in the glycerol on a balance, using density for glycerol = 1.26 g/ml). Mix the glycerol in by gentle vortexing. Incubate at 37¡C for 40'.
4. Layer on a warm cushion (10-14 ml) in two 50.2Ti (or equivalent) tubes. Spin for 45' at 40K at 35¡C in a 50.2 rotor.
5. Aspirate sample, rinse sample cushion interface with warm IX IB 2-3 times. Aspirate completely and rinse the tubulin pellet 2X with warm 1X IB to remove any residual glycerol. Do this while the pellet is held in a 37¡C bath -- fill tube ~half-way with warm IB (rinsing sides and pellet well), then aspirate and repeat. This greatly reduces glycerol and GTP contamination in the final tubulin stock.
6. Resuspend pellet in 1-2 ml of ice cold IB (the exact volume will depend on the concentration and polymerizability of the tubulin in the column fractions). Transfer the chunky pellet in IB to an ice-cold 2 ml dounce. Gently dounce on ice to break up the chunks. Incubate on ice for 30'. During this cold depolymerization, keep douncing gently every 2'-3'. Gentle sonication can also be used to break up the pellets.
7. Spin 90K in TLA100.2 or TLA100.3 rotor at 2¡C for 10'-15' to clarify the depolymerization mix.
8. Collect supernatant on ice and measure A280 of a 1/100 dilution in IB . Calculate concentration of tubulin using an extinction coefficient at 280 nm of 115,000 M-1 cm-1 . Freeze in 10-50 µl aliquots in liquid nitrogen and store at -80¡C. (Note: 1 mg/ml tubulin = 10 µM)