Affinity Purification of Soluble Lysosomal Proteins for Mass Spectrometric Identification
互联网
互联网
相关产品推荐

Recombinant-Hordeum-vulgare-High-molecular-mass-early-light-inducible-protein-HV58-chloroplasticHigh molecular mass early light-inducible protein HV58, chloroplastic; ELIP
¥10556

yscM/yscM蛋白/yscM; Yop proteins translocation protein M蛋白/Recombinant Yersinia enterocolitica Yop proteins translocation protein M (yscM)重组蛋白
¥69
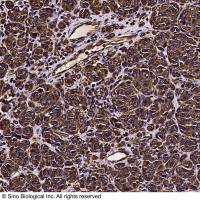
Vimentin Antibody, Rabbit PAb, Antigen Affinity Purified | Vimentin 兔多抗 (抗原亲和纯化)
¥1699

Recombinant-Mouse-Lysosomal-acid-phosphataseAcp2Lysosomal acid phosphatase; LAP EC= 3.1.3.2
¥12278

Recombinant-Hordeum-vulgare-Low-molecular-mass-early-light-inducible-protein-HV60-chloroplasticLow molecular mass early light-inducible protein HV60, chloroplastic; ELIP
¥9968
相关问答

