Determination of Affinity and Kinetic Rate Constants Using Surface Plasmon Resonance
互联网
互联网
相关产品推荐

TACSTD2/TACSTD2蛋白Recombinant Human Tumor-associated calcium signal transducer 2 (TACSTD2)重组蛋白Cell surface glycoprotein Trop-2 (Membrane component chromosome 1 surface marker 1) (Pancreatic carcinoma marker protein GA733-1)蛋白
¥1368

ROS试剂盒,用于样本中线粒体活性氧产生速率检测,ROS Production Rate Fluorometric Assay Kit
¥598

3,3′,5,5′-Tetramethylbenzidine Liquid Substrate, Slow Kinetic Form, for ELISA,peroxidase substrate,阿拉丁
¥815.90
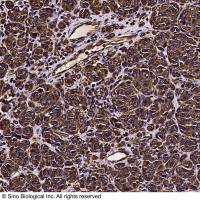
Vimentin Antibody, Rabbit PAb, Antigen Affinity Purified | Vimentin 兔多抗 (抗原亲和纯化)
¥1699

OPG105/OPG105蛋白/Carbonic anhydrase homolog蛋白/Recombinant Vaccinia virus Cell surface-binding protein OPG105 (OPG105)重组蛋白
¥69
相关问答

