Imaging -Galactosidase Activity In Vivo Using Sequential Reporter-Enzyme Luminescence
互联网
592
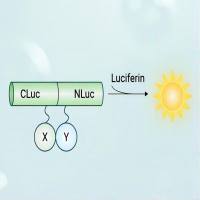
Bioluminescence using the reporter enzyme firefly luciferase (Fluc) and the substrate luciferin enables non-invasive optical imaging of living animals with extremely high sensitivity. This type of analysis enables studies of gene expression, tumor growth, and cell migration over time in live animals that were previously not possible. However, a major limitation of this system is that Fluc activity is restricted to the intracellular environment, which precludes important applications of in vivo imaging such as antibody labeling, or serum protein monitoring. In order to expand the application of bioluminescence imaging to other enzymes, we characterized a sequential reporter-enzyme luminescence (SRL) technology for the in vivo detection of β-galactosidase (β-gal) activity. The substrate is a “caged” d -luciferin conjugate that must first be cleaved by β-gal before it can be catalyzed by Fluc in the final, light-emitting step. Hence, luminescence is dependent on and correlates with β-gal activity. A variety of experiments were performed in order to validate the system and explore potential new applications. We were able to visualize non-invasively over time constitutive β-gal activity in engineered cells, as well as inducible tissue-specific β-gal expression in transgenic mice. Since β-gal, unlike Fluc, retains full activity outside of cells, we were able to show that antibodies conjugated to the recombinant β-gal enzyme could be used to detect and localize endogenous cells and extracellular antigens in vivo. In addition, we developed a low-affinity β-gal complementation system that enables inducible, reversible protein interactions to be monitored in real time in vivo, for example, sequential responses to agonists and antagonists of G-protein-coupled receptors (GPCRs). Thus, using SRL, the exquisite luminescent properties of Fluc can be combined with the advantages of another enzyme. Other substrates have been described that extend the scope to endogenous enzymes, such as cytochromes or caspases, potentially enabling additional unprecedented applications.