Characterization of Oligosaccharides from Starch, Dextran, Cellulose, and Glycoproteins by Capillary Electrophoresis
互联网
482
Neutral oligosaccharides are complex in terms of their linkage, either in the linear or branched form. An enormous number
of variants are therefore possible. A homopolymer of glucose with a glycosidic bond may assume its stere-ochemical configuration
at either the α or β position. Glucose units could be joined through 1,2; 1,3; 1,4; or 1,6 linkages in linear sequence or
extended through different linkages, which eventually could lead to a two- or three-dimensional network of homopolymers. Starch
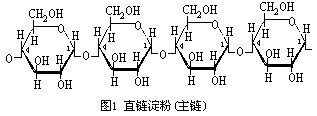
and dextran are homopolymer of glucose with α-1,4 and 1, 6 linkages, respectively, and are substantially different in physical
and chemical characteristics from the cellulose with the β-1,4 linkage. Because all neutral sugars do not have a charge and
seldom contain chromophores, methods for sugar analysis by capillary electrophoresis (CE) are thus limited in separation mechanism
as well as detection methods. Sugars do complex well with borate at high pH that provides the charge and yield significant
incremental absorbance at 195 nm but still with relatively low molar absorptivity (1
,2
). Alternatively, a chemical tag may be introduced to the reducing end of the neutral oligosaccharides to provide enhanced
sensitivity and introduce charges to the sugars for electrophoretic separation. The earliest CE-based analysis of derivatized
sugars was the reductive amination adducts of sugar with 2-aminopyridine (2-AP). The method was first explored by Honda et
al. (3
,4
) for high-resolution CE analysis of mono- and oligosaccharides.