
3D全自动外泌体荧光检测分析系统
研选同类产品更多 >
万千商家帮你免费找货
0 人在求购买到急需产品
- 详细信息
- 用户评价
- 文献和实验
- 技术资料
- 库存:
1
- 保修期:
面议
- 现货状态:
国内有样机
- 供应商:
Quantum量子科学仪器贸易(北京)有限公司
3D全自动外泌体荧光检测分析系统

法国Abbelight公司开发的3D全自动外泌体荧光检测分析系统是一款无需纯化的、全自动的可对单个外泌体进行表征分析的全新设备。该设备能够提供外泌体表征信息,包括外泌体粒径大小、亚型分布、携带蛋白表达、单个外泌体的膜蛋白与生物标志物共定位等。操作简单,结果可靠。
3D全自动外泌体荧光检测分析系统基于特异性免疫捕获技术,允许研究者直接分析特定群体的外泌体。通过单分子定位技术成像,可以得到单个外泌体的超分辨成像结果,尺度可以到20nm。全自动外泌体荧光检测分析系统兼容各种生物样本,除了纯化的外泌体之外,对于血液、尿液、恶性肿瘤、腹水中的外泌体也可直接检测分析,大大拓展了研究范围。
应用方向及主要特征
超高分辨单外泌体成像
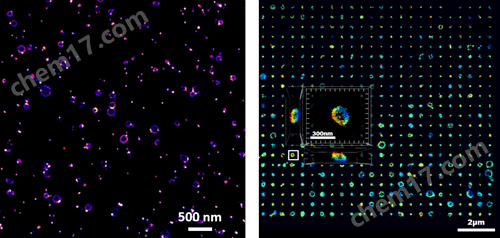
左:2D单个外泌体成像;右:3D单个外泌体成像
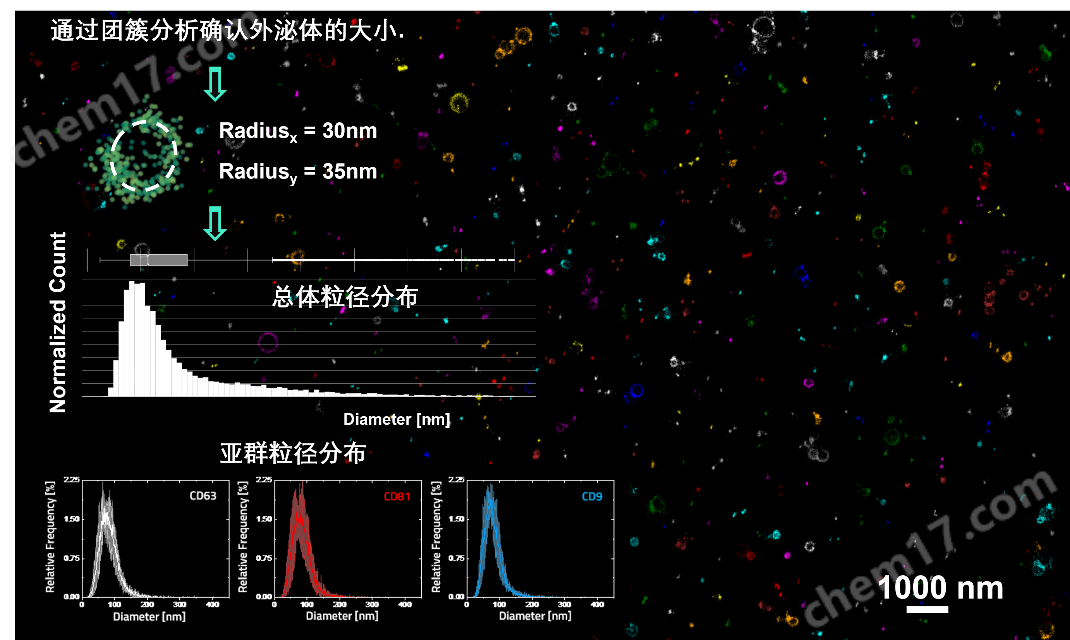
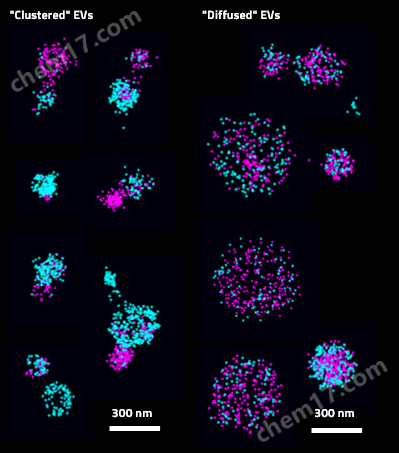
外泌体粒径分析:通过团簇分析外泌体的整体和亚群的粒径分布
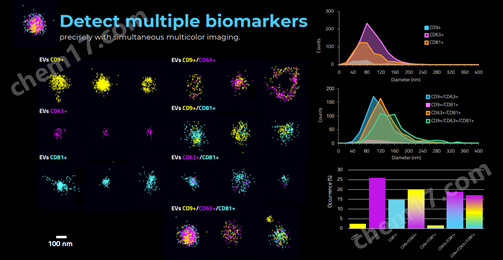
膜蛋白表征与共定位分析,以CD63,CD81&CD9为例表征其单阳,双阳和三阳的比例
外泌体内容物表征
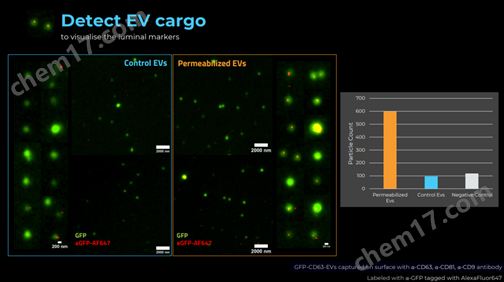
对比穿膜处理后外泌体中内容物aGFP的表达
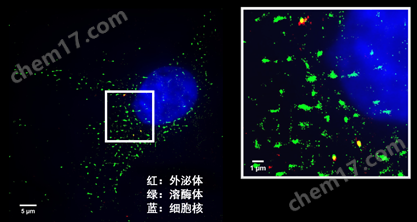
观测细胞中外泌体的分布情况,研究细胞中细胞器或者其他蛋白和外泌体的定位关系
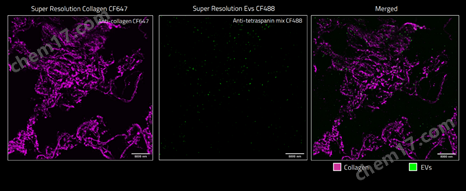
应用案例
法国Abbelight公司开发的3D全自动外泌体荧光检测分析系统是一款无需纯化的、全自动的可对单个外泌体进行表征分析的全新设备。该设备能够提供单个外泌体表征信息,包括外泌体粒径大小、亚型分布、携带蛋白表达、单个外泌体的膜蛋白与生物标志物共定位等。
应用领域:肿瘤诊断,药系统开发,眼科疾病诊断,疫苗研发,脊髓受伤机制研究,血浆/血清外泌体分析,外泌体工程化,呼吸疾病诊断......
测试数据
外泌体2D成像

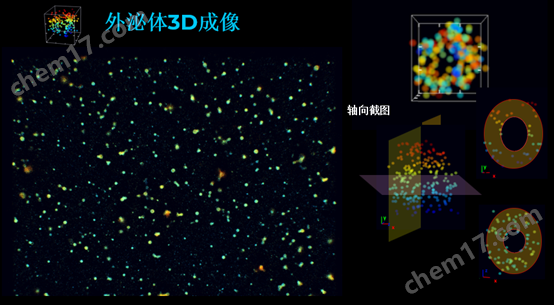
外泌体3D成像
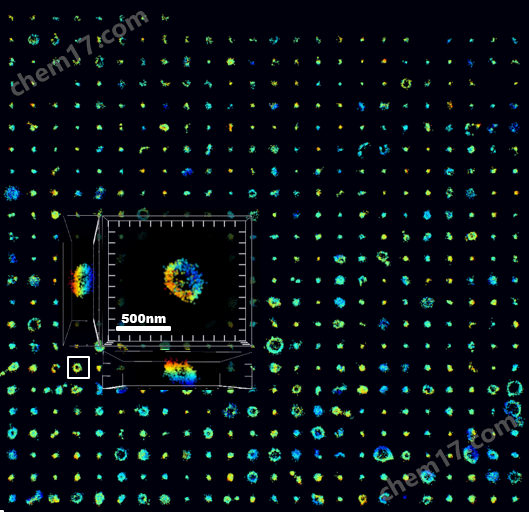
外泌体形态分析
用户单位

发表文章
[1] Storci, Gianluca, et al. "CAR+ extracellular vesicles predict ICANS in patients with B cell lymphomas treated with CD19-directed CAR T cells." The Journal of Clinical Investigation 134.14 (2024).
[2] Huang, Xiaowan, et al. "Nanoreceptors promote mutant p53 protein degradation by mimicking selective autophagy receptors." Nature Nanotechnology (2024): 1-9.
[3] Ye, Qian-Ni, et al. "Orchestrating NK and T cells via tri-specific nano-antibodies for synergistic antitumor immunity." Nature Communications 15.1 (2024): 6211.
[4] Shafaq-Zadah, Massiullah, et al. "Exploration into Galectin-3 Driven Endocytosis and Lattices." Biomolecules 14.9 (2024): 1169.
[5] Sanchez-Londono, Mariana, et al. "Visualization of Type IV-A1 CRISPR-mediated repression of gene expression and plasmid replication." Nucleic Acids Research 52.20 (2024): 12592-12603.
[6] Rajbanshi B, Guruacharya A, Mandell J W, et al. Localization, induction, and cellular effects of tau phosphorylated at threonine 217 1[J]. Alzheimer's & Dementia, 2023, 19(7): 2874-2887.
[7] Friedl, Karoline, et al. "Robust and fast multicolor Single Molecule Localization Microscopy using spectral separation and demixing." BioRxiv (2023): 2023-01.
[8] Baschieri, Francesco, et al. "Fibroblasts generate topographical cues that steer cancer cell migration." Science Advances 9.33 (2023): eade2120.
[9] Wessel, Aimee K., et al. "Escherichia coli SPFH membrane microdomain proteins HflKC contribute to aminoglycoside and oxidative stress tolerance." Microbiology Spectrum 11.4 (2023): e01767-23.
[10] Liu, Wei, et al. "Mitofusin-2 regulates leukocyte adhesion and β2 integrin activation." Journal of leukocyte biology 111.4 (2022): 771-791.
[11] Rajbanshi, Binita, et al. "Localization, induction, and cellular effects of tau phosphorylated at threonine 217." Alzheimer's & Dementia (2023).
[12] Pagliuca, M., et al. "38P Single molecule localization microscopy for extracellular vesicles detection in cancer." Annals of Oncology 33 (2022): S1395-S1396.
[13] Robaszkiewicz, A., and K. Gronkowska. "36P EP300 as an epigenetic target in p53 wild-type tumors treated with cisplatin." Annals of Oncology 33 (2022): S1395.
[14] He, Jin, et al. "Heterozygous Seryl‐tRNA Synthetase 1 Variants Cause Charcot–Marie–Tooth Disease." Annals of Neurology (2022).
[15] Gazzola, Morgan, et al. "Microtubules self-repair in living cells." Current Biology (2022).
[16] Liu, Wei, et al. "Mitofusin‐2 regulates leukocyte adhesion and β2 integrin activation." Journal of Leukocyte Biology 111.4 (2022): 771-791.
[17] Portes, Marion, et al. "Nanoscale architecture and coordination of actin cores within the sealing zone of human osteoclasts." Elife 11 (2022): e75610.
[18] Wessel, Aimee K., et al. "Escherichia coli membrane microdomain SPFH protein HflC interacts with YajC and contributes to aminoglycoside and oxidative stress tolerance." bioRxiv (2022).
[19] Radhakrishnan, A. V., et al. "Single-Protein Tracking to Study Protein Interactions During Integrin-Based Migration." The Integrin Interactome. Humana, New York, NY, (2021). 85-113.
[20] Jouchet, Pierre, et al. "Nanometric axial localization of single fluorescent molecules with modulated excitation." Nature Photonics (2021): 1-8.
[21] Orré, Thomas, et al. "Molecular motion and tridimensional nanoscale localization of kindlin control integrin activation in focal adhesions." Nature Communications 12.1 (2021): 3104.
[22] Pernier, Julien, et al. "Myosin 1b flattens and prunes branched actin filaments." Journal of cell science 133.18 (2020).
[23] Jimenez, Angélique, Karoline Friedl, and Christophe Leterrier. "About samples, giving examples: optimized single molecule localization microscopy." Methods 174 (2020): 100-114.
[24] Mau, Adrien, et al. "Fast scanned widefield scheme provides tunable and uniform illumination for optimized SMLM on large fields of view." bioRxiv (2020).
[25] Cabriel, Clément, et al. "Combining 3D single molecule localization strategies for reproducible bioimaging." Nature Communications 10.1 (2019): 1980.
[26] Woodhams, Stephen G., et al. "Cell type–specific super-resolution imaging reveals an increase in calcium-permeable AMPA receptors at spinal peptidergic terminals as an anatomical correlate of inflammatory pain." Pain 160.11 (2019): 2641-2650.
[27] Belkahla, Hanen, et al. "Carbon dots, a powerful non-toxic support for bioimaging by fluorescence nanoscopy and eradication of bacteria by photothermia." Nanoscale Advances (2019).
[28] Denis, Kevin, et al. "Targeting Type IV pili as an antivirulence strategy against invasive meningococcal disease." Nature microbiology 4.6 (2019): 972.
[29] Szabo, Quentin, et al. "TADs are 3D structural units of higher-order chromosome organization in Drosophila." Science advances 4.2 (2018): eaar8082.
[30] Boudjemaa, Rym, et al. "Impact of bacterial membrane fatty acid composition on the failure of daptomycin to kill Staphylococcus." Antimicrobial agents and chemotherapy 62.7 (2018): e00023-18.
风险提示:丁香通仅作为第三方平台,为商家信息发布提供平台空间。用户咨询产品时请注意保护个人信息及财产安全,合理判断,谨慎选购商品,商家和用户对交易行为负责。对于医疗器械类产品,请先查证核实企业经营资质和医疗器械产品注册证情况。
 用户评价
用户评价 暂无用户评价
暂无用户评价 文献和实验
文献和实验中国用户已发表文章
☛ 上海大学在《Journal of extracellular vesicles》发表文章。

☛ 中国科学院深圳技术研究院在《Lab on a Chip》发表文章。

☛ 北京天坛医院、国家纳米科学中心、北京航空航天大学在《Advanced Science》发表文章。

☛ 同济大学附属上海市肺科医院、上海思路迪转化医学在《Journal of Nanobiotechnology》发表文章。

☛ 山东千佛山医院在《NANO LETTERS》发表文章

感谢用户老师对Quantum Design China & NanoView设备的认可与支持!


国外部分已发表文献
1. Cytokine profiling in serum-derived exosomes isolated by different methods, Jung HH, Kim JY, Lim JE, Im YH, Nature Scientific Reports 2020
2. Study of immune-tolerized cell lines and extracellular vesicles inductive environment promoting continuous expression and secretion of HLA-G from semiallograft immune tolerance during pregnancy, Cho K, Kook H, Kang S Lee J, JEV 2020
3. Annexin A1–dependent tethering promotes extracellular vesicle aggregation revealed with single–extracellular vesicle analysis, Rogers MA, Buffolo F, Schlotter F, Atkins SK, Lee LH, Halu A, Blaser MC, Tsolaki E Higashi H, Luther K, Daaboul G, Bouten CVC, Body SC, Singh SA, Bertazzo S, Libby P, Aikawa M, Aikawa E, Cell Biology 2020
4. Targeting tumor-derived exosomes using a lectin affinity hemofiltration device, Marleau AM, Jacobs MT, Gruber N, Rodell TC, Ferrone S, Whiteside TL, Cancer Research 2020
5. Extracellular Vesicle and Particle Biomarkers DefineMultiple Human Cancers. Hoshino A, Kim HS, Bojmar L, Gyan KE, Cioffi M, Hernandez J, Zambirinis CP, Rodriques G, Molina H, Heissel S, Mark MT, Steiner L, Benito Martin A, Lucotti S, Di Giannatale A, Offer K, Nakajima M, Williams C, Lyden D., Cell 2020
6. Reporter mice for isolating and auditing cell type‐specific extracellular vesicles in vivo, McCann JV, Bischoff SR, Zhang Y, Cowley DO, Sanchez-Gonzalez V, Daaboul GG, Dudley AC, Genesis 2020
7. High yield and scalable EV production from suspension cells triggered by turbulence in a bioreactor, Grainger A, Wilhelm C, Gazeuah F, Silva A, Cytotherapy 2020
8. Extracellular Vesicles: A New Frontier for Research in Acute Respiratory Distress Syndrome, Mahida RY, Matsumoto S, Matthay MA. American Journal of Respiratory Cell and Molecular Biology 2020
9. Small extracellular vesicles modulated by αVβ3 integrin induce neuroendocrine differentiation in recipient cancer cells, Quaglia F, Krishn SR, Daaboul GG, Sarker S, Pippa R, Domingo-Domenech J, Kumar G, Fortina P, McCue P, Kelly WK, Beltran H, Liu Q, Languino LR., Journal of Extracellular Vesicles, Volume 9, Issue 1, 2020
10. Characterisation of extracellular vesicles surface markers and co-expression studies with single particle interferometric imaging platform, Kusuma G, Lim R., Cytotherapy, Volume 22, Issue 5, 2020
11. Engineering mesenchymal stem cell paracrine activity with 3D culture, Kusuma G, LiA, Zhu D, McDonald H, Chmabers D, Frith J, Lim R.,Cytotherapy, Volume 22, Issue 5, 2020
12. Release of extracellular vesicle miR-494-3p by ARPE-19 cells with impaired mitochondria,Ahn JY, Datta S, Cano M, Mallick E, Rai U, Powell B, Tian J, Witwer KW, Handa JT, Paulaitis ME.,Biochim Biophys Acta Gen Subj. 2020 Mar 30:129598. (doi: 10.1016/j.bbagen.2020.129598)
13. Advantageous Antibody Microarray Fabrication Through DNA-Directed Immobilization: A Step Toward Use of Extracellular Vesicles in Diagnostics, Brambila D, Sola L, Chiari M., Talanta 2020
14. Unannotated small RNA clusters in circulating extracellular vesicles detect early stage liver cancer, Villanueva et al, bioRxiv preprint 2020 (doi: https://doi.org/10.1101/2020.04.29.066183)
15. Membrane-Binding Peptides for Extracellular Vesicles On-Chip Analysis, Gori A, Romanato A, Bergamaschi G, Strada A, Gagni P, Frigerio R, Brambilla D, Vago R, Galbiati S, PIcciolini S, Bedoni M, Daaboul G, Chiari M, Cretich M., Journal of Extracellular Vesicles, Volume 9, Issue 1, 2020
16. Subpopulations of extracellular vesicles from human metastatic melanoma tissue identified by quantitative proteomics after optimized isolation, Crescitelli R, Lässer C, Jang SC, Cvjetkovic A, Malmhäll C, Karimi N, Höög JL, Johansson I, Fuchs J, Thorsell A, Gho YS, Bagge RO, Lötvall J, Journal of Extracellular Vesicles , 2020
17. Extracellular Vesicles Derived from Induced Pluripotent Stem Cells Promote Renoprotection in Acute Kidney Injury Model, Collino F, Lopes JA, Tapparo M, Tortelote GG, Kasai-Brunswick TH, Lopes GMC, Almeida DB, Skovronova R, Wendt CHC, de Miranda K, Bussolati B, Vieyra A, Soares Lindoso R., Cells, 2020
18. Extracellular Vesicles: A New Frontier for Research in Acute Respiratory Distress Syndrome,Mahida RY, Matsumoto S, Matthay MA. American Journal of Respiratory Cell and Molecular Biology, 2020
19. Exosomes A clinical COmpendium - Methods for exosome isolation and characterization,Zhou M, Weber SR, Zhao Y, Chan H, Sundstrom JM. Exosomes A Clinical Compendium 2020
20. Phenotypic analysis of extracellular vesicles: a review on the applications of fluorescence,Panagopoulou MS, Wark AW, Birch DJS, Gregory CD. Journal of Extracellular Vesicles, 2020
21. Immune Cell-Derived Exosomes in the Cancer-Immunity Cycle Yan W, Jiang S, Trends in Cancer 2020
, Colloids and Surfaces A: Physicochem. Eng. Aspects 387,2011, 35– 42. 相关产品: 1、ExoView外泌体全面表征试剂盒:https://www.biomart.cn/infosupply/99137706.htm 2、全自动外泌体荧光检测分析系统:https://www.biomart.cn/infosupply/94920084.htm
毒性T细胞杀伤 - 抗体依赖的细胞介导的细胞毒作用(ADCC) - 在肿瘤球模型中的免疫细胞杀伤 图2 采用Incucyte®活细胞分析系统分别检测免疫细胞对贴壁肿瘤细胞(SKOV-3,左图)、悬浮肿瘤细胞(WIL-NS,中图)和A549肿瘤球的杀伤作用(右图)。 Incucyte®免疫细胞 杀伤试验的主要优势 01 实时查看免疫细胞和肿瘤细胞之间的相互作用及杀伤过程,并进行全自动化的分析。 1. 观察并量化免疫细胞与肿瘤细胞之间的动态相互作用 2. 揭示细胞之间的相互作用,从而深入了解
和药敏板与全自动机型中的反应板是一样的。 5.抑菌圈直径测量仪 抑菌圈直径测量仪有BIOMIC(Giles Scientific Inc),AccuZone System (AccuMed International Inc) ,SIRSCAN (SIRSCAN) 等三种单板读取机,已在临床应用。经孵育后的药敏平板,被仪器的图象分析系统识别并计算抑菌圈直径。根据判断标准,报告药敏试验结果。这些仪器可减少人工测量抑菌圈直径大小差异及主观判断错误。BIOMIC系统宣称能根据抑菌圈大小
 技术资料
技术资料暂无技术资料 索取技术资料









