相关产品推荐更多 >
万千商家帮你免费找货
0 人在求购买到急需产品
- 详细信息
- 文献和实验
- 技术资料
- 英文名:
CEM/C1
- 库存:
100万
- 供应商:
欣润生物
- 肿瘤类型:
T淋巴母细胞
- 细胞类型:
细胞系
- ATCC Number:
详见说明
- 品系:
详见说明
- 组织来源:
外周血
- 相关疾病:
急性淋巴细胞白血病
- 物种来源:
人源
- 免疫类型:
不详
- 细胞形态:
淋巴母细胞样
- 是否是肿瘤细胞:
是
- 器官来源:
外周血
- 运输方式:
新鲜或干冰
- 年限:
成年
- 生长状态:
悬浮生长
- 规格:
T25方瓶
- 细胞名称:CEM/C1细胞(人急性淋巴细胞白血病细胞)
- 形态:淋巴母细胞样,贴壁生长
- 含量:>1x106 个/瓶
- 污染:支原体、细菌、酵母和真菌检测为阴性
- 规格:T25瓶或者1mL冻存管包装
二、细胞接收后的处理:
1、贴壁细胞
- 收到T25方瓶细胞后,请检查是否漏液,如果漏液,请拍照片发给我们(冻存管细胞收到后直接37℃水浴复苏或直接放置于液氮中长期储存)。
- 请先在显微镜下确认细胞生长状态,去掉封口膜并将T25瓶置于37℃培养约2-3h。
- 弃去T25瓶中的培养基,换用新鲜的完全培养基。
- 如果细胞长满(90%以上)请及时进行细胞传代。
- 接到细胞次日,请检查细胞是否污染,若发现污染或疑似污染,请及时与我们取得联系。
2、悬浮细胞
- 收到细胞后,请检查是否漏液,如果漏液,请拍照片发给我们。
- 请先在显微镜下确认细胞生长状态,去掉封口膜并将15ml离心管置于37℃培养约2-3h。
- 1200rpm离心5min,弃去15ml离心管中的培养基,细胞沉淀用新鲜的完全培养基重悬并培养。
- 如果细胞长满(90%以上)请及时进行细胞传代。
- 接到细胞次日,请检查细胞是否污染,若发现污染或疑似污染,请及时与我们取得联系。
本公司的细胞培养操作规程,供参考
一、培养基及培养冻存条件准备:
- 准备RPMI-1640培养基,90%;优质胎牛血清,10%。
- 培养条件: 气相:空气,95%;二氧化碳,5%。 温度:37℃,培养箱湿度为70%-80%。
- 冻存液:90%血清,10%DMSO,现用现配。液氮储存。
对于贴壁细胞,传代可参考以下方法:
- 弃去培养上清,用不含钙、镁离子的PBS润洗细胞1-2次。
- 加2ml消化液(0.25%Trypsin-0.53mM EDTA)于培养瓶中,置于37℃培养箱中消化2-3分钟,然后在显微镜下观察细胞消化情况,若细胞大部分变圆并脱落,迅速拿回操作台,轻敲几下培养瓶后加入3ml此细胞的培养基终止消化。
- 轻轻吹打后吸出,移入15ml离心管中,在1200RPM条件下离心5分钟,弃去上清液,加入1mL培养液后吹匀。
- 移入到事先准备好的含有5ml培养基的T-25培养瓶中或含有14ml培养基的T-75培养瓶中培养。
3)细胞冻存:待细胞生长状态良好时,可进行细胞冻存。贴壁细胞冻存时,先要消化处理并进行细胞计数。消化方法按照细胞传代方法的1-3步骤进行,最后的重悬液使用血清。悬浮细胞直接计数后离心,用血清重悬浮,加DMSO至最终浓度为10%。加入DMSO后迅速混匀,按每1ml的数量分配到冻存管中。本公司按每个冻存管细胞数目大于1X106个细胞冻存。
注意事项:
1. 收到冻存管细胞后,若发现干冰已挥发干净、冻存管瓶盖脱落、破损及细胞有污染,请立即与我们联系。
2. 所有动物细胞均视为有潜在的生物危害性,必须在二级生物安全台内操作,并请注意防护,所有废液及接触过此细胞的器皿需要灭菌后方能丢弃。
3. 细胞用途:仅供科研使用。
发货方式:
复苏后发货:我们复苏细胞后发货,货期一周左右,免运费。(气温较好建议复苏后发货)
冻存发货(干冰运输):需额外增加干冰运费,选择干冰运输的我们发两管细胞,为了保证客户接种可靠性多发一管。(气温低于0℃须冻存发货)
细胞发货采取专业的运输包装,并选择最快捷的运输方式(顺丰速运或其他空运快递)
Resistance to glucocorticoid-induced apoptosis in human T-cell acute lymphoblastic leukemia CEM-C1 cells is due to insufficient glucocorticoid receptor expression
The ability of glucocorticoids (GCs) to induce death in lymphoid-origin cells is the basis for their frequent use in the therapy of various human hematological malignancies. However, the occurrence of primary or secondary GC resistance limits their clinical usefulness. Prior investigations into the mechanism of GC resistance in established human leukemic cell lines revealed loss-of-function mutations in the GC receptor (GR) gene. In this study, we analyzed the GC-resistant human acute T-cell leukemia line CEM-C1, which has been reported to express biochemically functional GR and, thus, was thought to owe its GC resistance to signal transduction changes distal from the GR. Radioligand binding assays revealed a 2-3-fold lower expression of GR in CEM-C1 than in the GC-sensitive sister cell line CEM-C7H2. Analysis of transcriptional activity using mouse mammary tumor virus-long terminal repeat-controlled chloramphenicol acetyltransferase expression in transient transfection assays confirmed the expression of functional GR in CEM-C1 but at levels lower than those in CEM-C7H2 cells. Upon molecular analyses of the GR gene and its transcripts, we found that CEM-C1 cells were heterozygous for the ligand binding domain L753F point mutation in exon 9, which is also present in GC-sensitive CEM-C7H2. No mutations, however, were found on the second GR allele of CEM-C1. To test the possibility that resistance in CEM-C1 cells might be caused by insufficient expression of GR, we established several cell lines stably transfected with rat GR expression vectors. These cell lines differed in exogenous GR expression as determined by Northern blotting and radioligand binding assays. The GR expression level in individual lines correlated well with their sensitivity to GC-induced apoptosis. Thus, GC resistance of CEM-C1 cells might be due to subthreshold expression of functional GR rather than defects in signal transduction pathways distal from the GR. Since several clinical investigations showed a correlation between reduced GR expression and poor response to GC-containing treatment, the CEM-C1 line may represent a valid model for GC resistance in human acute T-cell leukemia.
Growth inhibitory effects of bioflavonoids and related compounds on human leukemic CEM-C1 and CEM-C7 cells
even compounds, which included some naturally occurring dietary substances, were tested for their inhibitory effects on growth and metabolism of human leukemic CEM-C1 and CEM-C7 cell lines. Among the active compounds the naturally occurring dietary constituents were found to be the most active. The strongest inhibitory effects were observed with 3′,4′,5,7-tetrahydroxy-flavone (luteolin) and 4,4′-dihydroxychalcone. 31 P-NMR spectra of cells incubated for 24 h with 30 μM of either of these compounds show complete ATP depletion. Also glucose uptake by the cells as measured by 13 C-NMR is completely inhibited by these compounds. These results may be relevant to the tumor suppressing activity of bioflavonoids and the role of these compounds in chemoprevention.
风险提示:丁香通仅作为第三方平台,为商家信息发布提供平台空间。用户咨询产品时请注意保护个人信息及财产安全,合理判断,谨慎选购商品,商家和用户对交易行为负责。对于医疗器械类产品,请先查证核实企业经营资质和医疗器械产品注册证情况。
 文献和实验
文献和实验Chicken intestinal epithelial cells were obtained from NEWGAINBIO company. Cells were cultured on 37℃, with 5% CO2, in the Ham’s F-12 Nutrient (DMEM/12) that contained the following supplementations: fetal bovine serum (5%), in-sulin (5 µg/mL), transferrin (5 µg/mL), selenium (5 ng/mL), epidermal growth factor (5 ng/mL) and penicillin-streptomycin (100–100 U/mL) for cell culturing (full DMEM/12). Experiments were performed with chicken intestinal epithelial cells and working solutions were prepared with plain DMEM/12 without supplementation. For the investigations, cells were seeded onto 96-well, 24-well or 6-well polystyrene cell culture plates.
Primary hVICs (passage 2) were cultured to 50–60% confluence and infected with pGMLV-SV40T-puro lentivirus (NewgainBio, Wuxi, China) at a multiplicity of infection of 80 supplemented with 5 µg/mL polybrene (Sigma-Aldrich, Buchs, Switzerland).
Tissue was cultured until cells became visible around the tissue, and when the fusion reached 90% (FIGURE 1A) §ask ¦lled with the prepared culturing medium was sent to the company for further immortalisation. Cell immortalisation was done for cell stability and longer-term use. Immortalised cells were cultured with 10% FBS and 1% PS in the DMEM medium. After the cells multiplied and merged, they were routinely passed and grown ( NEWGAINBIO Inc. Wuxi, Jiangsu, China) (FIGURE 1B-C).
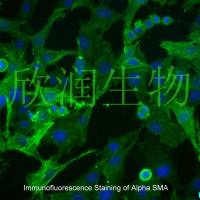
Mouse primary cultured renal vascular ECs and VSMCs were obtained from Newgainbio company, which were tested by Factor VIII and α-smooth muscle actin (α-SMA), the marker of ECs and VSMCs. RNeasy Mini Kit was used for RNA extraction, and the above protocols were repeated.
Porcine primary colon epithelial cells (Newgainbio company, Wuxi,China) were cultured in Dulbecco's Modified Eagle's Medium (Solarbio, Beijing, China) containing 10 % fetal bovine serum (BioInd, Kiryat shmona, Lsrael) at 37 ◦C and 5 % CO2 humidity.
病 AtT20 垂体瘤 P388D1 淋巴样瘤 NS-1 骨髓瘤 SRS-82 腹水瘤 SP2/0 骨髓瘤 SAC-IIB2 腹水瘤 P3-NS-1/1-Ag4.1 骨髓瘤 SAC-IIC3 腹水瘤 45.6.TG1.7 骨髓瘤 S180 腹水瘤 P3-X63-Ag8 骨髓瘤 B16 黑色素瘤 J774A.1 单核细胞-巨噬细胞 C127 乳腺肿瘤 RAW264.7 单核细胞-巨噬细胞 F9 胚胎瘤 NG108-15 小鼠神经细胞瘤×大鼠神经胶质细胞杂交细胞2. 大鼠类 C6 胶质瘤 RH-35
瘤 45.6.TG1.7 骨髓瘤 S180 腹水瘤 P3-X63-Ag8 骨髓瘤 B16 黑色素瘤 J774A.1 单核细胞-巨噬细胞 C127 乳腺肿瘤 RAW264.7 单核细胞-巨噬细胞 F9 胚胎瘤 NG108-15 小鼠神经细胞瘤×大鼠神经胶质细胞杂交细胞 2.大鼠类 C6 胶质瘤 RH-35 肝癌 SHZ-88 乳腺癌 CBRH-7919 肝癌 PC-12 肾上腺嗜铬细胞瘤 3.人类 *A431
/+/q0s8fX9J0KCtH7EtiZy60HdVrVJceMfUh+FwlIe3hgwd49eoVTp86Beu9+xASEgINDQ0YkEBSqWIlNGveHHXr1c1AWpKoNAK+vcZ3uqSqoUZiBuXBebIW4ybYo9rqu9jdLk+k/wd0TOPxh2rmhH5O/uiPSpFfKZoAvwMVTTwjjhf8FRc22cLHcBS6lzFGzn7VMWecDbbbTUD5+jnC82d4XxmEWv0d0XRqI7xbsxP2f1OB5m86D5vHamL