相关产品推荐更多 >
万千商家帮你免费找货
0 人在求购买到急需产品
- 详细信息
- 文献和实验
- 技术资料
- 库存:
247
- 英文名:
Pibrentasvir
- CAS号:
1353900-92-1
- 保质期:
2年
- 供应商:
北京百奥莱博科技有限公司
- 保存条件:
-20℃,有效期2年,溶入溶剂后-20℃
- 规格:
1mL(10mM)|5mg|10mg|25mg|50mg|100mg
特别提示:包括HCV NS5A抑制剂(Pibrentasvir)在内,本公司的所有产品仅可用于科研实验,严禁用于临床医疗及其他非科研用途!
产品名称:HCV NS5A抑制剂(Pibrentasvir)
英文名称:Pibrentasvir
CAS#:1353900-92-1
产品货号:M03935
产品规格:1mL(10mM)|5mg|10mg|25mg|50mg|100mg
Pibrentasvir是一种新型的泛基因型丙型肝炎病毒(HCV)NS5A抑制剂,对来自基因型1至6含有NS5A的HCV复制子的EC50范围为1.4至5.0pM。
注:本品仅可用于科研实验,严禁用于临床医疗及其他用途!
CAS号:1353900-92-1
别名:ABT-530
纯度:99.52%
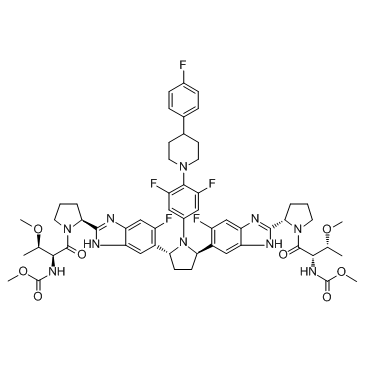
分子式:C₅₇H₆₅F₅N₁₀O₈
分子量:1113.18
结构式:
储存条件:-20℃,有效期2年,溶入溶剂后-20℃请尽量在一个月内使用。
除了HCV NS5A抑制剂(Pibrentasvir),ABT-530,我公司还供应以下相关产品:
| 货号 | 名称 | 规格 |
| M00161 | p53抑制剂(芳香烃受体激动剂) | 1mL(10mM)|5mg|10mg|25mg|50mg |
| M00919 | 泛Ras抑制剂(泛RAS-IN-1) | 1mL(10mM)|1mg|5mg|10mg|25mg|50mg|100mg |
| M01025 | 多靶点激酶抑制剂(KW-2449) | 1mL(10mM)|5mg|10mg|50mg|100mg |
| M01615 | VEGFR抑制剂(KRN-633) | 1mL(10mM)|5mg|10mg|50mg|100mg |
| M01659 | MGMT抑制剂(Lomeguatrib) | 1mL(10mM)|10mg|50mg |
| M01709 | PI3Kα抑制剂(GDC-0326) | 1mL(10mM)|5mg|10mg|25mg|50mg|100mg |
| M02201 | c-Met抑制剂(AMG-208) | 5mg|10mg|50mg|100mg |
| M02233 | GnRH激动剂(Buserelin Acetate) | 1mL(10mM)|2mg|5mg|10mg|50mg|100mg |
| M02812 | fXa可逆型抑制剂(Otamixaban) | 1mL(10mM)|10mg|100mg |
| M05232 | CPT-1抑制剂(Etomoxir) | 1mL(10mM)|5mg|10mg|50mg |
| M05918 | NAMPT激活剂(P7C3) | 1mL(10mM)|5mg|10mg|50mg|100mg |
风险提示:丁香通仅作为第三方平台,为商家信息发布提供平台空间。用户咨询产品时请注意保护个人信息及财产安全,合理判断,谨慎选购商品,商家和用户对交易行为负责。对于医疗器械类产品,请先查证核实企业经营资质和医疗器械产品注册证情况。
 文献和实验
文献和实验Studying HCV Cell Entry with HCV Pseudoparticles (HCVpp)
HCV infection leads in 50 to 80% of cases to chronic hepatitis, liver cirrhosis, or hepatocellular carcinoma. Interferons and the nucleoside analog ribavirin form the basis for treatment but are not sufficiently effective and have numerous
Simultaneous Detection of Anti-HCV Antibody and HCV Core Antigen
HCV infection is usually diagnosed by means of an enzyme immune assay for the detection of antibody against HCV. The window period between infection and seroconversion remains a dramatic problem in the transfusional and diagnostic setting
The AMPLICOR HCV Tests for the Detection and Quantitation of Serum or Plasma HCV RNA
Clinical diagnosis of HCV infection is generally accomplished by using immunoserological assays to detect the presence of anti-HCV antibodies. Such immunoserological assays have been approved for blood donor screening, thereby reducing
 技术资料
技术资料暂无技术资料 索取技术资料