相关产品推荐更多 >
万千商家帮你免费找货
0 人在求购买到急需产品
- 详细信息
- 询价记录
- 文献和实验
- 技术资料
- 抗体名:
InVivoMab anti-mouse PD-1 (CD279)
- 抗体英文名:
InVivoMab anti-mouse PD-1 (CD279)
- 靶点:
请见产品说明
- 浓度:
请见产品说明
- 应用范围:
in vivo blocking of PD-1/PD-L signaling
- 宿主:
请见产品说明
- 适应物种:
mouse
- 供应商:
欣博盛生物
- 标记物:
无
- 克隆性:
多克隆
- 保存条件:
4度避光
- 形态:
液体
- 亚型:
Rat IgG2a
- 免疫原:
mouse PD-1 (CD279)
- 规格:
5 mg
InVivoMab anti-mouse PD-1 (CD279) 货号:BE0146-5MG ,还有其它四种规格可供选择。如需购买产品,或咨询技术问题,请联系Bioxcell授权代理欣博盛生物~
BioXcell热销产品--InVivoMab anti-mouse PD-1 (CD279)
产品描述:
BioXcell InVivoMab anti-mouse PD-1 RMP1-14单克隆抗体与小鼠PD-1(也称为CD279)反应。PD-1是一种50-55kDa的细胞表面受体,由Pdcd1基因编码,属于Ig超家族的CD28家族。PD-1在CD4和CD8胸腺细胞以及活化的T和B淋巴细胞和髓细胞上瞬时表达,在成功消除抗原后PD-1的表达下降。此外,在B细胞前阶段,Pdcd1 mRNA在发育中的B淋巴细胞中表达。PD-1的结构包括ITIM(基于免疫受体酪氨酸的抑制基序),这表明PD-1负调控TCR信号。PD-1通过结合B7家族的成员PD-L1和PD-L2发出信号。在配体结合后,PD-1信号传导抑制T细胞活化,导致增殖减少,细胞因子产生和T细胞死亡。此外,PD-1敲除动物表现出扩张型心肌病、脾肿大和外周耐受丧失,在小鼠的外周耐受性和预防自身免疫性疾病中发挥关键作用。诱导的PD-L1表达常见于许多肿瘤,包括鳞状细胞癌、结肠癌和乳腺癌。PD-L1过度表达会导致肿瘤细胞对CD8T细胞介导的裂解的抗性增加。在黑素瘤的小鼠模型中,可以通过用阻断PD-L1与其受体PD-1之间相互作用的抗体治疗来暂时抑制肿瘤生长。由于这些原因,目前正在探索抗PD-1介导的免疫疗法作为癌症治疗。与J43抗体一样,RMP1-14抗体已显示阻断小鼠PD-L1-Ig和小鼠PD-L2-Ig与PD-1的结合。
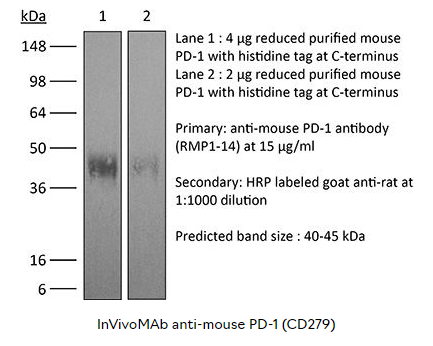
产品详情:
| 产品名称 |
InVivoMAb anti-mouse PD-1 (CD279) |
| 产品货号 |
BE0146 |
| 产品规格 |
1/5/25/50/100mg |
| 反应种属 |
Mouse |
| 克隆号 |
RMP1-14 |
| 同种型 |
Rat IgG2a, κ |
| 免疫原 |
Syrian Hamster BKH cells transfected with mouse PD-1 cDNA |
| 实验应用 |
in vivo blocking of PD-1/PD-L signaling |
| 产品形式 |
PBS, pH 7.0,Contains no stabilizers or preservatives |
| 纯度 |
>95%, Determined by SDS-PAGE |
| 无菌处理 |
0.2 µm filtration |
| 纯化方式 |
Protein G |
| RRID |
AB_10949053 |
| 分子量 |
150 kDa |
| 保存条件 |
抗体原液保存在4°C,不能冷冻保存。 |
| 推荐同型对照 |
InVivoMAb rat IgG2a isotype control, anti-trinitrophenol(货号BE0089) |
| 推荐抗体稀释液 |
InVivoPure pH 7.0 Dilution Buffer(货号IP0070) |
该产品自上市已被多篇SCI文献引用,品质有保证,以下是部分已发表的文献引用:
| 应用 |
文章 |
| 体内PD-1/PD-L信号阻断 (in vivo blocking of PD-1/PD-L signaling) |
1. Triplett, T. A., et al. (2018). 'Reversal of indoleamine 2,3-dioxygenase-mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme' Nat Biotechnol 36(8): 758-764. 2. Grasselly, C., et al. (2018). 'The Antitumor Activity of Combinations of Cytotoxic Chemotherapy and Immune Checkpoint Inhibitors Is Model-Dependent' Front Immunol 9: 2100. 3. Moynihan, K. D., et al. (2016). 'Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses' Nat Med. doi : 10.1038/nm.4200. 4. Ngiow, S. F., et al. (2015). 'A Threshold Level of Intratumor CD8+ T-cell PD1 Expression Dictates Therapeutic Response to Anti-PD1' Cancer Res 75(18): 3800-3811. 5. Evans, E. E., et al. (2015). 'Antibody Blockade of Semaphorin 4D Promotes Immune Infiltration into Tumor and Enhances Response to Other Immunomodulatory Therapies' Cancer Immunol Res 3(6): 689-701. 6. Zelenay, S., et al. (2015). 'Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity' Cell 162(6): 1257-1270. 7. Zander, R. A., et al. (2015). 'PD-1 Co-inhibitory and OX40 Co-stimulatory Crosstalk Regulates Helper T Cell Differentiation and Anti-Plasmodium Humoral Immunity' Cell Host Microbe 17(5): 628-641.
|

更多产品详情请咨询 BioXcell 中国授权代理——欣博盛生物
风险提示:丁香通仅作为第三方平台,为商家信息发布提供平台空间。用户咨询产品时请注意保护个人信息及财产安全,合理判断,谨慎选购商品,商家和用户对交易行为负责。对于医疗器械类产品,请先查证核实企业经营资质和医疗器械产品注册证情况。
- 作者
- 内容
- 询问日期
 文献和实验
文献和实验in vivo blocking of PD-1/PD-L signaling
Triplett, T. A., et al. (2018). "Reversal of indoleamine 2,3-dioxygenase-mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme" Nat Biotechnol 36(8): 758-764. PubMed
Increased tryptophan (Trp) catabolism in the tumor microenvironment (TME) can mediate immune suppression by upregulation of interferon (IFN)-gamma-inducible indoleamine 2,3-dioxygenase (IDO1) and/or ectopic expression of the predominantly liver-restricted enzyme tryptophan 2,3-dioxygenase (TDO). Whether these effects are due to Trp depletion in the TME or mediated by the accumulation of the IDO1 and/or TDO (hereafter referred to as IDO1/TDO) product kynurenine (Kyn) remains controversial. Here we show that administration of a pharmacologically optimized enzyme (PEGylated kynureninase; hereafter referred to as PEG-KYNase) that degrades Kyn into immunologically inert, nontoxic and readily cleared metabolites inhibits tumor growth. Enzyme treatment was associated with a marked increase in the tumor infiltration and proliferation of polyfunctional CD8(+) lymphocytes. We show that PEG-KYNase administration had substantial therapeutic effects when combined with approved checkpoint inhibitors or with a cancer vaccine for the treatment of large B16-F10 melanoma, 4T1 breast carcinoma or CT26 colon carcinoma tumors. PEG-KYNase mediated prolonged depletion of Kyn in the TME and reversed the modulatory effects of IDO1/TDO upregulation in the TME.
in vivo blocking of PD-1/PD-L signaling
Grasselly, C., et al. (2018). "The Antitumor Activity of Combinations of Cytotoxic Chemotherapy and Immune Checkpoint Inhibitors Is Model-Dependent" Front Immunol 9: 2100. PubMed
In spite of impressive response rates in multiple cancer types, immune checkpoint inhibitors (ICIs) are active in only a minority of patients. Alternative strategies currently aim to combine immunotherapies with conventional agents such as cytotoxic chemotherapies. Here, we performed a study of PD-1 or PDL-1 blockade in combination with reference chemotherapies in four fully immunocompetent mouse models of cancer. We analyzed both the in vivo antitumor response, and the tumor immune infiltrate 4 days after the first treatment. in vivo tumor growth experiments revealed variable responsiveness to ICIs between models. We ob-served enhanced antitumor effects of the combination of immunotherapy with chemotherapy in the MC38 colon and MB49 bladder models, a lack of response in the 4T1 breast model, and an inhibition of ICIs activity in the MBT-2 bladder model. Flow cytometry analysis of tumor samples showed significant differences in all models between untreated and treated mice. At baseline, all the tumor models studied were predominantly infiltrated with cells harboring an immunosuppressive phenotype. Early alterations of the tumor immune infiltrate after treatment were found to be highly variable. We found that the balance between effector cells and immunosuppressive cells in the tumor microenvironment could be altered with some treatment combinations, but this effect was not always correlated with an impact on in vivo tumor growth. These results show that the combination of cytotoxic chemotherapy with ICIs may result in enhanced, similar or reduced antitumor activity, in a model- and regimen-dependent fashion. The present investigations should help to select appropriate combination regimens for ICIs.
in vivo blocking of PD-1/PD-L signaling
Moynihan, K. D., et al. (2016). "Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses" Nat Med. doi : 10.1038/nm.4200. PubMed
Checkpoint blockade with antibodies specific for cytotoxic T lymphocyte-associated protein (CTLA)-4 or programmed cell death 1 (PDCD1; also known as PD-1) elicits durable tumor regression in metastatic cancer, but these dramatic responses are confined to a minority of patients. This suboptimal outcome is probably due in part to the complex network of immunosuppressive pathways present in advanced tumors, which are unlikely to be overcome by intervention at a single signaling checkpoint. Here we describe a combination immunotherapy that recruits a variety of innate and adaptive immune cells to eliminate large tumor burdens in syngeneic tumor models and a genetically engineered mouse model of melanoma; to our knowledge tumors of this size have not previously been curable by treatments relying on endogenous immunity. Maximal antitumor efficacy required four components: a tumor-antigen-targeting antibody, a recombinant interleukin-2 with an extended half-life, anti-PD-1 and a powerful T cell vaccine. Depletion experiments revealed that CD8+ T cells, cross-presenting dendritic cells and several other innate immune cell suB-SEts were required for tumor regression. Effective treatment induced infiltration of immune cells and production of inflammatory cytokines in the tumor, enhanced antibody-mediated tumor antigen uptake and promoted antigen spreading. These results demonstrate the capacity of an elicited endogenous immune response to destroy large, established tumors and elucidate essential characteristics of combination immunotherapies that are capable of curing a majority of tumors in experimental settings typically viewed as intractable.
in vivo blocking of PD-1/PD-L signaling
Ngiow, S. F., et al. (2015). "A Threshold Level of Intratumor CD8+ T-cell PD1 Expression Dictates Therapeutic Response to Anti-PD1" Cancer Res 75(18): 3800-3811. PubMed
Despite successes, thus far, a significant proportion of the patients treated with anti-PD1 antibodies have failed to respond. We use mouse tumor models of anti-PD1 sensitivity and resistance and flow cytometry to assess tumor-infiltrating immune cells immediately after therapy. We demonstrate that the expression levels of T-cell PD1 (PD1(lo)), myeloid, and T-cell PDL1 (PDL1(hi)) in the tumor microenvironment inversely correlate and dictate the efficacy of anti-PD1 mAb and function of intratumor CD8(+) T cells. In sensitive tumors, we reveal a threshold for PD1 downregulation on tumor-infiltrating CD8(+) T cells below which the release of adaptive immune resistance is achieved. In contrast, PD1(hi) T cells in resistant tumors fail to be rescued by anti-PD1 therapy and remain dysfunctional unless intratumor PDL1(lo) immune cells are targeted. Intratumor Tregs are partly responsible for the development of anti-PD1-resistant tumors and PD1(hi) CD8(+) T cells. Our analyses provide a framework to interrogate intratumor CD8(+) T-cell PD1 and immune PDL1 levels and response in human cancer. Cancer Res; 75(18); 3800-11. (c)2015 AACR.
in vivo blocking of PD-1/PD-L signaling
Evans, E. E., et al. (2015). "Antibody Blockade of Semaphorin 4D Promotes Immune Infiltration into Tumor and Enhances Response to Other Immunomodulatory Therapies" Cancer Immunol Res 3(6): 689-701. PubMed
Semaphorin 4D (SEMA4D, CD100) and its receptor plexin-B1 (PLXNB1) are broadly expressed in murine and human tumors, and their expression has been shown to correlate with invasive disease in several human tumors. SEMA4D normally functions to regulate the motility and differentiation of multiple cell types, including those of the immune, vascular, and nervous systems. In the setting of cancer, SEMA4D-PLXNB1 interactions have been reported to affect vascular stabilization and transactivation of ERBB2, but effects on immune-cell trafficking in the tumor microenvironment (TME) have not been investigated. We describe a novel immunomodulatory function of SEMA4D, whereby strong expression of SEMA4D at the invasive margins of actively growing tumors influences the infiltration and distribution of leukocytes in the TME. Antibody neutralization of SEMA4D disrupts this gradient of expression, enhances recruitment of activated monocytes and lymphocytes into the tumor, and shifts the balance of cells and cytokines toward a proinflammatory and antitumor milieu within the TME. This orchestrated change in the tumor architecture was associated with durable tumor rejection in murine Colon26 and ERBB2(+) mammary carcinoma models. The immunomodulatory activity of anti-SEMA4D antibody can be enhanced by combination with other immunotherapies, including immune checkpoint inhibition and chemotherapy. Strikingly, the combination of anti-SEMA4D antibody with antibody to CTLA-4 acts synergistically to promote complete tumor rejection and survival. Inhibition of SEMA4D represents a novel mechanism and therapeutic strategy to promote functional immune infiltration into the TME and inhibit tumor progression.
这种水果将助力癌症治疗?增强免疫治疗效果,有望克服 PD-1 耐药难题!
素 C、花青素类物质,已被证明可以通过增加肠道中嗜粘液杆菌(A.muciniphila)和双歧杆菌(Bifidobacterium)的丰度,对小鼠的肥胖和相关代谢紊乱发挥保护性作用。 2022 年 1 月 14 日,来自加拿大蒙特利尔大学研究中心的 Bertrand Routy 团队在 Cancer Discovery 上发表了题为 A natural polyphenol exerts antitumor activity and circumvents anti-PD-1 resistance
分别利用 CD3 与 CD28 的功能学抗体,以及 CD3/CD28 Streptamer® 激活扩增 T 细胞的实验步骤与实验结果。 CD3/CD28抗体法 1、实验步骤: 1.1 抗体包被 用无菌 PBS 将 anti-mouse CD3 抗体(克隆号:145-2C11)稀释至 5μg/ml,稀释后的抗体加入到 24 孔板中,每孔 400μl,4°C 包被过夜。(注:板子在加入抗体之前,先用无菌 PBS 润洗 2-3 遍,避免干孔); 1.2 小鼠脾脏淋巴细胞分离(次日) 1. 将 70μm
Purification of Immature CD4+CD8+ Thymocytes by Panning with Anti-CD8 Antibody
Most T lymphocytes of the immune system differentiate within the thymus along the CD4/CD8 developmental pathway by a highly ordered process termed thymic selection ( 1 , 2 ). The maturation status of thymocytes is commonly assessed
 技术资料
技术资料暂无技术资料 索取技术资料







