万千商家帮你免费找货
0 人在求购买到急需产品
- 详细信息
- 询价记录
- 文献和实验
- 技术资料
- 供应商:
ATCC
- 细胞类型:
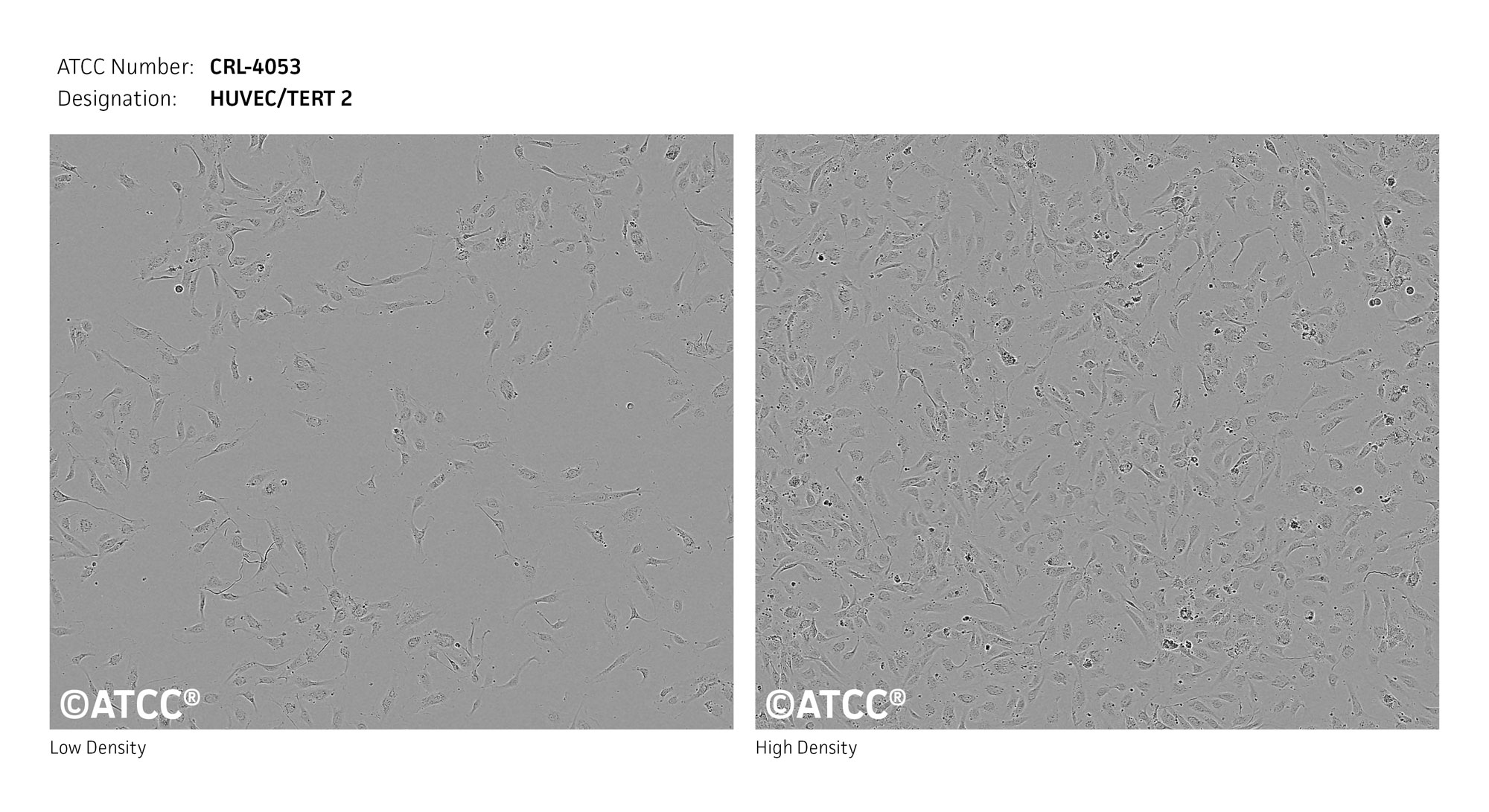
Endothelial cell immortalized with hTERT
- ATCC Number:
CRL-4053
- 组织来源:
Umbilical vascular endothelium
- 相关疾病:
Normal
- 物种来源:
Homo sapiens, human
- 细胞形态:
Cobblestone appearance with large dark nuclei
- 运输方式:
干冰运输
- 年限:
neonate
- 生长状态:
贴壁
- 英文名:
HUVEC/TERT 2; Human Umbilical; Vein Endothelial Cell; Human (Homo sapiens)
- 规格:
1ml/支
| Organism | Homo sapiens, human |
|---|---|
| Tissue | Umbilical vascular endothelium |
| Cell Type | Endothelial cell immortalized with hTERT |
| Product Format | frozen 1 mL |
| Morphology | Cobblestone appearance with large dark nuclei |
| Culture Properties | adherent |
| Biosafety Level | 2 [These primary cells are not known to harbor an agent recognized to cause disease in healthy adult humans. Handle as a potentially biohazardous material under at least Biosafety Level 1 containment. Cells derived from primate lymphoid tissue may fall under the regulations of 29 CFR 1910.1030 Bloodborne Pathogens.
ATCC recommends that appropriate safety procedures be used when handling all primary cells and cell lines, especially those derived from human or other primate material. Detailed discussions of laboratory safety procedures are provided in Laboratory Safety: Principles and Practice, 2nd ed. (ASM Press, Washington, DC) (Fleming et al., 1995) and Caputo, J.L. Biosafety procedures in cell culture. (1988) J. Tissue Culture Methods 11:223. Appropriate safety procedures should always be used with this material. Laboratory safety is discussed in the following publication: Biosafety in Microbiological and Biomedical Laboratories, 5th ed. HHS Publication No. (CDC) 93-8395. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Washington DC: U.S. Government Printing Office; 2007] Human Material Precaution All tissues used for isolation are obtained under informed consent and conform to HIPAA standards to protect the privacy of the donor’s personal health information. It is best to use caution when handling any human cells. We recommend that all human cells be accorded the same level of biosafety consideration as cells known to carry HIV. With infectious virus assays or viral antigen assays, even a negative test result may leave open the possible existence of a latent viral genome. Biosafety classification is based on U.S. Public Health Service Guidelines, it is the responsibility of the customer to ensure that their facilities comply with biosafety regulations for their own country. |
| Disease | Normal |
| Age | Newborn |
| Gender | female |
| Ethnicity | Caucasian |
| Applications | Immune response, wound healing, cellular response to viral or bacterial infection, oxidative stress, angiogenesis, arteriolosclerosis, drug screening, tubule formation |
| Shipping Information | Frozen 1 mL |
| Storage Conditions | -130°C |
风险提示:丁香通仅作为第三方平台,为商家信息发布提供平台空间。用户咨询产品时请注意保护个人信息及财产安全,合理判断,谨慎选购商品,商家和用户对交易行为负责。对于医疗器械类产品,请先查证核实企业经营资质和医疗器械产品注册证情况。
- 作者
- 内容
- 询问日期
 文献和实验
文献和实验Optimized Fibrin Gel Bead Assay for the Study of Angiogenesis
PREPARING CELLS Bring up HUVEC and fibroblasts in M199/10% FBS/Pen-Strep (1:100) 1-2 days before beading. Switch medium to EGM-2 (Clonetics) the day before beading for HUVEC and the day before embedding for fibroblasts.
PRODUCTION OF COMPLETELY ES CELL-DERIVED FETUSES BY AGGREGATION WITH TETRAPLOID
tip) alternatively: Finger controlled pipette (Manostat tubing, yellow tip, scalpel blade); 9'' Pasteur pipettes; 70% Ethanol; Alcohol burner or Bunsen burner; Sterile plastic Petri dishes (100 x 15 mm); Sterile
(-9) HUVEC 0.35 0.51 1.2 X 10(-8) 4.2 X 10(-7) 表 2:检测开发与评价概述
 技术资料
技术资料暂无技术资料 索取技术资料