相关产品推荐更多 >
万千商家帮你免费找货
0 人在求购买到急需产品
- 详细信息
- 文献和实验
- 技术资料
- 库存:
100
- 供应商:
上海酶研生物科技有限公司
- 英文名:
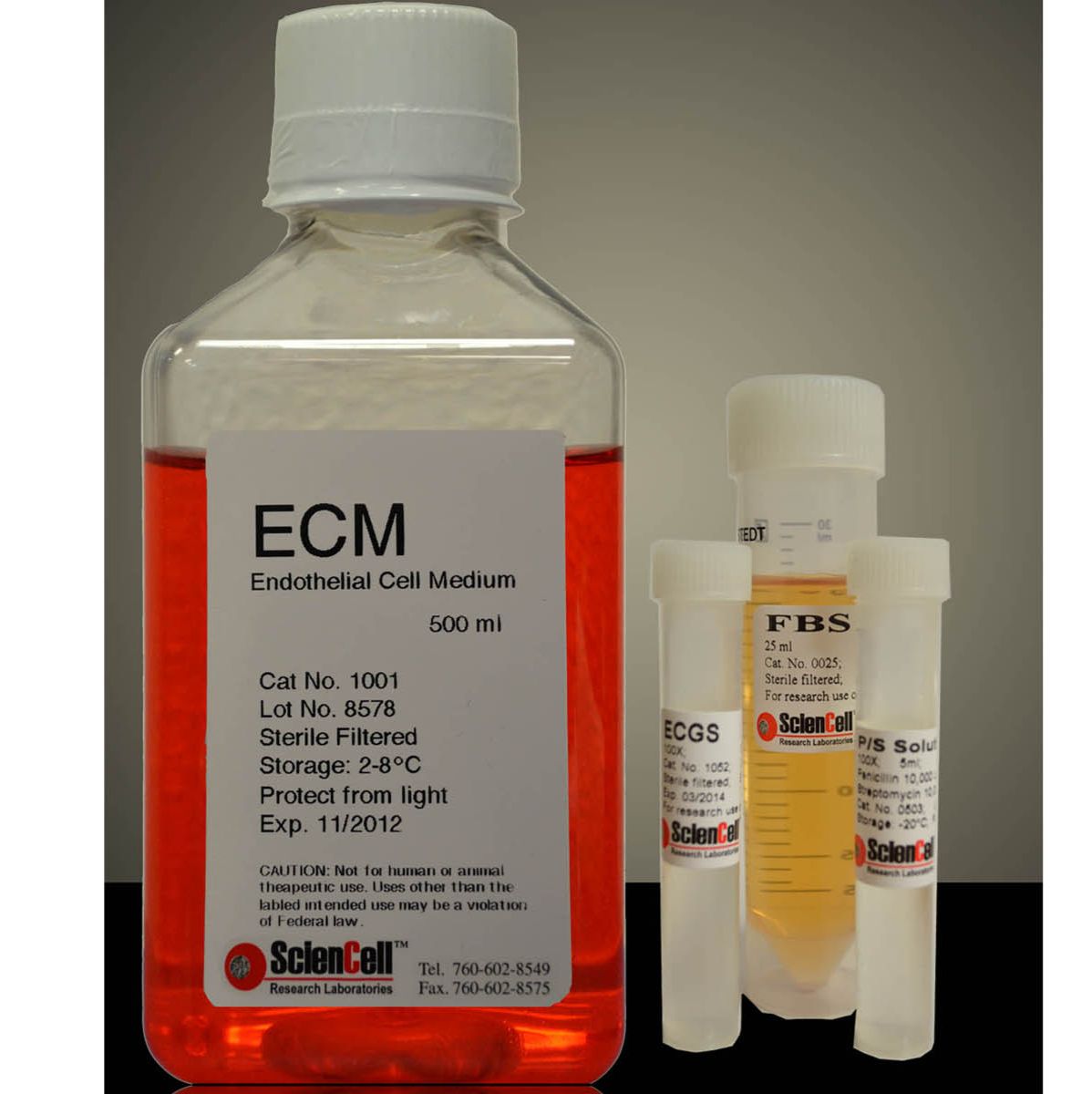
ObM (Osteoblast Medium)
- 规格:
500ml/T
|
货号 |
4601 |
|
产地 |
美国 |
|
缩写 |
ObM |
|
规格 |
500ml |
|
用途 |
科研 |
|
储存 |
4度,-20度 |
|
运输 |
胶冰 |
成骨细胞培养基是为正常人类成骨细胞体外培养设计的适于其生长的培养基。是经灭菌的液体培养基,包含必需和非必需氨基酸、维生素、有机和无机化合物、激素、生长因子、微量矿物质和低浓度胎牛血清(5%)。该培养基缓冲体系为重碳酸盐,在含5%CO2的细胞培养箱中平衡后pH值为7.4。该培养基的配方能够选择性的促进正常人类成骨细胞体外培养中的增殖和生长,并为其达到理想营养平衡状态提供数量上和质量上的保证。
成骨细胞培养基包含500 ml基础培养基,10ml胎牛血清(FBS,目录编号0025),5ml成骨细胞生长添加物(ObGS,目录编号4652)和5 ml青霉素/链霉素溶液(P/S,目录编号0503)
1. Liu, X.L., Hu, X., Cai, W.X., Lu, W.W. & Zheng, L.W. (2016) Effect of Granulocyte-Colony Stimulating Factor on Endothelial Cells and Osteoblasts Biomed Res Int. 2016
Objectives. Some animal studies showed that granulocyte-colony stimulating factor (G-CSF) provides beneficial environment for bone healing. It has been well documented that endothelial cells and osteoblasts play critical roles in multiple phases of bone healing. However, the biological effects of G-CSF on these cells remain controversial. This study aimed to investigate the influence of G-CSF at various concentrations on endothelial cells and osteoblasts. Materials and Methods. Human umbilical vein endothelial cells (HUVECs) and human osteoblasts (hOBs) were treated with G-CSF at 1000, 100, 10, and 0 ng/mL, respectively. The capacity of cell proliferation, migration, and tube formation of HUVECs was evaluated at 72, 8, and 6 hours after treatment, respectively. The capacity of proliferation, differentiation, and mineralization of hOBs was evaluated at 24 hours, 72 hours, and 21 days after treatment, respectively. Results. HUVECs treated with 100 and 1000 ng/mL G-CSF showed a significantly higher value comparing with controls in migration assay (p < 0.001, p < 0.01, resp.); the group treated with 1000 ng/mL G-CSF showed a significantly lower value on tube formation. No significant difference was detected in groups of hOBs. Conclusions. G-CSF showed favorable effects only on the migration of HUVECs, and no direct influence was found on hOBs. Less
2. Tran N, Tran PA, Jarrell JD, Engiles JB, Thomas NP, Young MD, Hayda RA, Born CT. (2013) "In Vivo Caprine Model for Osteomyelitis and Evaluation of Biofilm-Resistant Intramedullary Nails." Biomed Res Int. 2013: 674378 doi: 10.1155/2013/674378
Bone infection remains a formidable challenge to the medical field. The goal of the current study is to evaluate antibacterial coatings in vitro and to develop a large animal model to assess coated bone implants. A novel coating consisting of titanium oxide and siloxane polymer doped with silver was created by metal-organic methods. The coating was tested in vitro using rapid screening techniques to determine compositions which inhibited Staphylococcus aureus growth, while not affecting osteoblast viability. The coating was then applied to intramedullary nails and evaluated in vivo in a caprine model. In this pilot study, a fracture was created in the tibia of the goat, and Staphylococcus aureus was inoculated directly into the bone canal. The fractures were fixed by either coated (treated) or non-coated intramedullary nails (control) for 5 weeks. Clinical observations as well as microbiology, mechanical, radiology, and histology testing were used to compare the animals. The treated goat was able to walk using all four limbs after 5 weeks, while the control was unwilling to bear weight on the fixed leg. These results suggest the antimicrobial potential of the hybrid coating and the feasibility of the goat model for antimicrobial coated intramedullary implant evaluation. Less
风险提示:丁香通仅作为第三方平台,为商家信息发布提供平台空间。用户咨询产品时请注意保护个人信息及财产安全,合理判断,谨慎选购商品,商家和用户对交易行为负责。对于医疗器械类产品,请先查证核实企业经营资质和医疗器械产品注册证情况。
 文献和实验
文献和实验
1.) Tran N, Tran PA, Jarrell JD, Engiles JB, Thomas NP, Young MD, Hayda RA, Born CT. (2013) "In Vivo Caprine Model for Osteomyelitis and Evaluation of Biofilm-Resistant Intramedullary Nails." Biomed Res Int. 2013: 674378 doi: 10.1155/2013/674378
实验材料: 1. 骨组织来源:新生或胚胎大鼠、兔、人类胚胎或手术切除之骨组织; 2. 清洗液:不含Ca2+ 和Mg2+ 的1×PBS,添加100IU/ml青霉素、100μg/ml链霉素,pH7.2; 3. 消化液:0.25%胰蛋白酶溶液,1mg/mlⅡ型胶原酶溶液; 4. 培养液:RPMI 1640培养基,配制培养液时加入15%的小牛血清,并用5.6% NaHCO3 调节至pH 7.2。 实验方法: 1. 取生后1—2d大鼠若干只,拉颈处死
实验材料: 1. 骨组织来源:手术切除之骨组织; 2. 清洗液:不含Ca2+ 和Mg2+ 的1×PBS,添加100IU/ml青霉素、100μg/ml链霉素,pH7.2; 3. 消化液:0.25%胰蛋白酶溶液,1mg/mlⅠ型胶原酶溶液; 4. 培养液:RPMI 1640培养基,配制培养液时加入15%的小牛血清,并用5.6% NaHCO3 调节至pH 7.2. 实验方法:
我曾经做过原代培养的成骨细胞传代和骨髓基质细胞以及cos7细胞的传代。基本的操作过程都是:倒掉或者吸掉培养基37度预温的PBS洗涤细胞3次加入浓度为0.1%的胰酶和0.05%EDTA进行消化,添加的量看所用的培养瓶大小,可以在室温下消化,也可以在37度进行消化在显微镜下观察,等细胞成片脱离时,加入3毫升含血清培养基中止消化将细胞悬液转移到离心管中1000rpm离心5分钟收集细胞弃上清,再加入5毫升含血清培养基重悬细胞,再同样转速和时间离心一次弃上清,根据实际需要添加适量培养基重悬
 技术资料
技术资料暂无技术资料 索取技术资料