相关产品推荐更多 >
万千商家帮你免费找货
0 人在求购买到急需产品
- 详细信息
- 文献和实验
- 技术资料
- 品系:
详见细胞说明资料
- 细胞类型:
详见细胞说明资料
- 肿瘤类型:
详见细胞说明资料
- 供应商:
上海冠导生物工程有限公司
- 库存:
≥100瓶
- 生长状态:
详见细胞说明资料
- 年限:
详见细胞说明资料
- 运输方式:
常温运输【复苏细胞】或干冰运输【冻存细胞】
- 器官来源:
详见细胞说明资料
- 是否是肿瘤细胞:
详见细胞说明资料
- 细胞形态:
详见细胞说明资料
- 免疫类型:
详见细胞说明资料
- 物种来源:
详见细胞说明资料
- 相关疾病:
详见细胞说明资料
- 组织来源:
详见细胞说明资料
- 英文名:
T24[T-24]人膀胱移行细胞癌传代细胞长期复苏|送STR图谱
- 规格:
1*10(6)Cellls/瓶
"T24[T-24]人膀胱移行细胞癌传代细胞长期复苏|送STR图谱
传代方法:1:2-1:4(首次传代建议1:2)
生长特性:贴壁生长
换液频率:每周2-3次
背景资料:该细胞源自一位81岁白人女性患者的膀胱移行细胞癌组织;来源于移行细胞癌病人的白血病和血浆对T24和相关细胞株有细胞毒性;倍增时间为19小时;含ras(H-ras)癌基因,表达肿瘤特有抗原。
关于细胞株是否都能一直传代,答案是否定的。细胞系分为有限细胞系与连续细胞系两类。有限细胞系就像一位有着既定行程的行者,其传代之旅存在明确的终点。以正常的人体肝细胞系为例,在体外培养时,它大约能传代20-30次。随着传代次数的递增,细胞内部仿佛一台精密仪器的零件逐渐磨损老化。端粒酶活性降低,端粒不断缩短,染色体结构开始不稳定,基因表达也出现异常。同时,细胞的代谢速率减缓,对营养物质的摄取和利用效率大打折扣,有害物质的积累却日益增多,最终导致细胞停止分裂,走向生命的尽头。而连续细胞系则像是拥有无限活力的长跑健将,具有较强的传代能力。如Hela细胞系,自1951年从Henrietta Lacks女士的宫颈癌组织中分离出来后,便在全球生物实验室中“大放异彩”,至今已传代无数次。它能持续分裂得益于其特殊的遗传变异,使其染色体端粒能够维持稳定长度,并且细胞内的一些关键信号通路持续激活,促进细胞增殖。但这并不意味着它的传代毫无风险与限制。在漫长的传代过程中,它可能会发生新的基因突变、染色体易位等变异事件,从而改变细胞的生物学特性,如细胞形态、生长速度、对药物的敏感性等。
┈订┈购┈热┈线:1┈5┈8┈0┈0┈5┈7┈6┈8┈6┈7【微信同号】┈Q┈Q:3┈3┈0┈7┈2┈0┈4┈2┈7┈1;
GM07483 Cells(拥有STR基因鉴定图谱)
NCI-H1650 Cells;背景说明:该细胞是从一名27岁白人男性(10年烟龄)支气管肺泡癌患者的胸腔积液中分离得到的。;传代方法:1:4-1:6传代;2-3天换液1次。;生长特性:贴壁生长;形态特性:上皮细胞样;相关产品有:P30/0HK Cells、Normal fibroblast-10 Cells、SKRC-42 Cells
CL-34 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:MDA134 Cells、IOSE-29 Cells、Hep 3B Cells
T24[T-24]人膀胱移行细胞癌传代细胞长期复苏|送STR图谱
产品包装形式:复苏细胞:T25培养瓶(一瓶)或冻存细胞:1ml冻存管(两支)
来源说明:细胞主要来源ATCC、DSMZ等细胞库
悬浮细胞不容易转染:悬浮细胞是指细胞生长不依赖支持物表面,在培养中呈悬浮状态生长,如淋巴细胞。在实验室经常会遇到悬浮细胞的转染,其和贴壁细胞转染还是有很大不同的。目前大多数实验室用的是脂质体类的转染试剂,脂质体转染是基于电荷吸引原理,先形成脂质体-DNA复合物,散布在细胞周围,然后通过细胞的内吞作用,将目的基因导入细胞内,而脂质体复合物与贴壁细胞的接触机会比悬浮细胞GAO出很多倍,所以,脂质体转染时悬浮细胞的转染效率要明显低于贴壁细胞。其次,脂质体试剂的毒性较大,这就使得悬浮细胞的转染更为困难了。另外,悬浮细胞不易培养,易死亡,也给细胞转染造成了一定的困难。为了提GAO悬浮细胞的转染效率,可以使用非脂质体的转染试剂,如纳米材料的。也可以使用电转染方法,针对悬浮细胞等难转染细胞还是挺不错的,电击对细胞有一定的损伤,ZuiHAO选用具有细胞膜修复功能的电转染试剂,可以将电击对细胞的伤害降到Zui低,悬浮细胞不易转染,选对试剂及良HAO的细胞培养环境才是关键。
物种来源:Human\Mouse\Rat\Others
293 H Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:RGC5 Cells、NCI-H2291 Cells、H-125 Cells
GM07404D Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长 ;形态特性:详见产品说明;相关产品有:H1838 Cells、NS20Y Cells、KP2 Cells
RSC-364 Cells;背景说明:滑膜 Cells;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:NCI-H-128 Cells、KMBC Cells、MES-SA Cells
T24[T-24]人膀胱移行细胞癌传代细胞长期复苏|送STR图谱
形态特性:上皮细胞样
┈订┈购┈热┈线:1┈5┈8┈0┈0┈5┈7┈6┈8┈6┈7【微信同号】┈Q┈Q:3┈3┈0┈7┈2┈0┈4┈2┈7┈1;
细胞根据其形态和功能可以分为多种类型,其中上皮细胞、成纤维细胞和淋巴母细胞是常见的三种类型。成纤维细胞,或称纤维母细胞,是一种存在于蜂窝组织或纤维结缔组织中的梭形细胞,能够分泌构成细胞外基质(ECM)的结构蛋白。这类细胞最初由德国病理学家鲁道夫·菲尔绍与法国解剖学家马蒂亚斯·杜瓦尔于19世纪中叶描述。成纤维细胞特异性标志物包括FAP(成纤维细胞激活蛋白)、α-SMA(α-平滑肌肌动蛋白)、骨膜蛋白、PDGFRα(血小板衍生生长因子受体α)、胶原蛋白、波形蛋白、纤连蛋白和胸腺细胞抗原1等。成纤维细胞在形态上是扁平的纺锤形细胞,缺乏基底膜,细胞核呈圆形拉长,被巨大的内质网包围。在培养中,它们表现出不同的形态学和生化特征,这取决于其起源位置、分化状态和培养条件。
CHO-K1 Cells;背景说明:1957年,PuckTT从成年中国仓鼠卵巢的活检组织建立了CHO细胞,CHO-K1是CHO的一个亚克隆。CHO-K1的生长需要脯酸。;传代方法:1:2传代;生长特性:贴壁生长;形态特性:上皮样;相关产品有:V 79 Cells、MCF 7 Cells、NCI-HUT-69 Cells
NCI-H378 Cells;背景说明:小细胞肺癌;胸腔积液转移;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:WRL-68 Cells、RC-4B Cells、KHYG Cells
FL-83B Cells;背景说明:肝;自发永生;C57BL/6J;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:SW954 Cells、Hi-5 Cells、MDA175 Cells
JB6 Cl 30 Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长;形态特性:上皮样;相关产品有:MD Anderson-Metastatic Breast-435 Cells、HEY-A8 Cells、NCIH1184 Cells
L615 Cells;背景说明:白血病;615小鼠;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:悬浮;形态特性:详见产品说明;相关产品有:GM02132C Cells、KOSC-2 Cells、Earles's cells Cells
U118-MG Cells;背景说明:注意: 据报道来自不同个体的胶质母细胞瘤细胞株U-118 MG (HTB-15) 和 U-138 MG (HTB-16)有着一致的VNTR和相近的STR模式。 U-118 MG 和 U-138 MG细胞遗传学上很相似并有至少六个衍生标记染色体。 这是1966年至1969年间J. Ponten和同事从恶性神经胶质瘤中构建的细胞株中的一株(其它包括ATCC HTB-14和 ATCC HTB-16 and ATCC HTB-17)。 1987年用BM-Cycline培养6周去除了支原体污染。 ;传代方法: 消化3-5分钟。1:2传代。3天内可长满。;生长特性:贴壁生长;形态特性:混合型;相关产品有:SV-HUC Cells、brain-derived Endothelial cells.3 Cells、GH 3 Cells
BHP 10-3 Cells;背景说明:甲状腺乳头状癌;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:W133 Cells、Jurkat clone A3 Cells、RPMI 1788 Cells
NCI-SNU-761 Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:293 Cells、KM12 Cells、SNU-182 Cells
HPDE Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长 ;形态特性:详见产品说明;相关产品有:PC2 Cells、CTSC-3 Cells、P3X63 AG 8.653 Cells
FHCRC-11 Cells;背景说明:该细胞源自一位14岁患有T淋巴细胞白血病男性的外周血;传代方法:保持细胞密度在3—9×105cells/ml之间,1:5—1:10传代,每周换液2—3次;生长特性:悬浮生长;形态特性:圆形,单个或呈片;相关产品有:Metastatic Variant-522 Cells、SupT1 Cells、NCI-H2227 Cells
CCLP1 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:HS0578T Cells、P3X63-Ag8.653 Cells、F442-A Cells
OCM-1 Cells;背景说明:葡萄膜黑色素瘤;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:BV-173 Cells、GM03320D Cells、Mv1.Lu Cells
alpha-TC1-6 Cells;背景说明:胰岛素瘤;a细胞;C57BL/6xDBA/2;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:SW-527 Cells、CTV-1 Cells、SLK Cells
HBEpiC Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:RWPE-2 Cells、SKO3 Cells、GM03571 Cells
AD293 Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长;形态特性:详见产品说明;相关产品有:T-T Cells、MV4:11 Cells、ID8/MOSEC Cells
QGY 7701 Cells;背景说明:详见相关文献介绍;传代方法:消化3-5分钟,1:2,3天内可长满;生长特性:贴壁生长;形态特性:上皮样;相关产品有:NCI H23 Cells、HOPC Cells、MS1 Cells
H596 Cells;背景说明:详见相关文献介绍;传代方法:1:4-1:8传代;每周换液2-3次。;生长特性:贴壁生长;形态特性:上皮样;相关产品有:GA-10(Clone 4) Cells、CCD 19Lu Cells、Dakiki Cells
Abcam A-549 CBL KO Cells(拥有STR基因鉴定图谱)
Abcam Raji TPP2 KO Cells(拥有STR基因鉴定图谱)
BayGenomics ES cell line BGC386 Cells(拥有STR基因鉴定图谱)
BayGenomics ES cell line RRS151 Cells(拥有STR基因鉴定图谱)
BayGenomics ES cell line YTA400 Cells(拥有STR基因鉴定图谱)
Chem-9 GLP1R Cells(拥有STR基因鉴定图谱)
DA01890 Cells(拥有STR基因鉴定图谱)
F15553 Cells(拥有STR基因鉴定图谱)
PNT1-a Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:DHL4 Cells、GC-1 Cells、MSTO-211 Cells
KM12-SM Cells;背景说明:结肠癌;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:143B TK- Cells、Potorous tridactylus Kidney 2 Cells、HLFa Cells
T24[T-24]人膀胱移行细胞癌传代细胞长期复苏|送STR图谱
MSTO211H Cells;背景说明:MSTO-211H细胞株是1985年从一位肺二相间皮瘤患者的胸水中建株的。这个病人接受过多种药物联合前期化疗。MSTO-211H细胞具有高亲和力的EGF结合位点,并表达神经元特异性烯醇酶(NSE)及人绒毛膜促性腺激素(HCG)的α与β亚基。未检测到左旋多巴胺脱羧酶(DDC),邦巴辛与神经tensin。细胞过表达c-myc原癌基因,并没有观察到基因重排或扩增。V-src,v-abl,v-erbB,c-raf1,Ha-ras,Ki-ras,和N-ras的表达呈阳性。未检测到N-m;传代方法:消化3-5分钟。1:2。3天内可长满。;生长特性:贴壁生长;形态特性:成纤维细胞样;相关产品有:NFS-60 Cells、SUM-159PT Cells、CATH-a Cells
VMM39 Cells;背景说明:黑色素瘤;神经节转移;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:A498 Cells、SK-ML2 Cells、KCL22 Cells
HG2855 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:P116 Cells、H-735 Cells、MLE-12 Cells
SW 480 Cells;背景说明:SW480源自一位51岁白人男性患者的原位直肠腺癌,而SW620源自同一病人一年后的淋巴结转移灶。该细胞CSAp和直肠抗原3阴性;角蛋白阳性;p53基因第273位密码子的G→A突变引起Arg→His替代,309位密码子的C→T突变导致Pro→Ser替代;细胞p53蛋白表达水平升高;癌基因c-myc、K-ras、H-ras、N-ras、myb、sis和fos的表达呈阳性;未检测到癌基因N-myc的表达;不表达Matrilysin(一种与肿瘤侵袭相关的金属蛋白酶)。;传代方法:1:2传代,1-2天换液一次;生长特性:贴壁生长;形态特性:上皮样;相关产品有:Balb/c 3T3 Cells、CAL120 Cells、NCI/ADR-RES Cells
VP 229 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:COLO-680N Cells、BTI-Tn-5B1-4 Cells、UM-UC3 Cells
NCI-H322 Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长;形态特性:详见产品说明;相关产品有:CEM-T4 Cells、MCA205 Cells、BT 20 Cells
AZc6#22 Cells(拥有STR基因鉴定图谱)
TSCC1 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:SW-756 Cells、M4e Cells、HuP-T4 Cells
HC11 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:MM6 Cells、HEC-251 Cells、NUGC4 Cells
┈订┈购┈热┈线:1┈5┈8┈0┈0┈5┈7┈6┈8┈6┈7【微信同号】┈Q┈Q:3┈3┈0┈7┈2┈0┈4┈2┈7┈1;
Panc 08.13 Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长;形态特性:上皮样;相关产品有:AZ 521 Cells、HKF Cells、HSAS1 Cells
ECC1 Cells;背景说明:内膜腺癌;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:MC3T3-E1 Subclone 24 Cells、GM-3573 Cells、DKMG Cells
GA-10 clone 4 Cells;背景说明:详见相关文献介绍;传代方法:每2-3天换液;生长特性:悬浮生长 ;形态特性:淋巴母细胞样;相关产品有:P388.D1 Cells、Hs 636 T Cells、SNU-869 Cells
HUT 125 Cells;背景说明:腺鳞状肺癌;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:LTEP a2 Cells、TPC1 Cells、SW260 Cells
HUT 125 Cells;背景说明:腺鳞状肺癌;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:LTEP a2 Cells、TPC1 Cells、SW260 Cells
High5 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:C2BBe 1 Cells、Kit225 K6 Cells、BrCL15 Cells
GT38 Cells(拥有STR基因鉴定图谱)
HAP1 PRKAG1 (-) 1 Cells(拥有STR基因鉴定图谱)
A2008 Cells;背景说明:宫颈鳞癌;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:OsACL Cells、AU-Mel Cells、H-35 Reuber Cells
H-2291 Cells;背景说明:详见相关文献介绍;传代方法:1:3-1:4传代;每周换液2-3次。;生长特性:贴壁生长;形态特性:上皮细胞样;相关产品有:SuDHL 4 Cells、BCaP-37 Cells、HeLa/DDP Cells
KGN Cells;背景说明:该细胞株是颗粒细胞瘤。细胞在加入人体绒膜促性腺激素后可能产生孕酮。细胞生长缓慢。;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:上皮细胞样;相关产品有:SO-RB50 Cells、HS688AT Cells、SKCO1 Cells
J774 A1 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:Hs 895.T Cells、C-26 Cells、OCIAML3 Cells
LNCaP-Clone-FGC Cells;背景说明:人前列腺癌细胞LNCaP克隆FGC是从一位50岁白人男性(血型B+)的左锁骨淋巴结针刺活检中分离,该患者经确诊为前列腺癌转移。 这株细胞对5-α-二睾酮(生长调节子和酸性脂酶产物)有响应。这株细胞并不形成一致的单层,而是形成集落,在传代时可以用滴管反复吹吸打碎。它们仅仅轻轻地吸附在基底上,不形成汇合,很快使培养基变酸。生长很慢。传代后48小时内不应扰动。当培养瓶封包后,多数细胞从培养瓶底分离,悬浮在培养基中。收到后,在通常培养单层细胞的条件下培养24到48小时,以合细胞再贴壁。;传代方法:消化3-5分钟。1:2。3天内可长满。;生长特性:贴壁生长;形态特性:上皮细胞;相关产品有:H1954 Cells、3T6 Swiss Albino Cells、Tj-905 Cells
MDST8 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:NCIH2286 Cells、A-704 Cells、MBdSMC Cells
Hep G2-Luc Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:SUDHL-5 Cells、A673 Cells、Ls-174-T Cells
Centre Antoine Lacassagne-62 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:MESSA Cells、IHC-ST1 Cells、CEM-CCRF (CAMR) Cells
HUES 37 Cells(拥有STR基因鉴定图谱)
KYSE-360 Cells(拥有STR基因鉴定图谱)
MS-LG Cells(拥有STR基因鉴定图谱)
OCUCh-LM1 Cells(拥有STR基因鉴定图谱)
RMO1 Cells(拥有STR基因鉴定图谱)
Ubigene HCT 116 TNFRSF13C KO Cells(拥有STR基因鉴定图谱)
WAe025-A-1 Cells(拥有STR基因鉴定图谱)
HeLa S3 AGO2KO Cells(拥有STR基因鉴定图谱)
293A Cells;背景说明:胚肾;腺病毒包装;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:WC00044 Cells、A-2058 Cells、4175 Cells
HO-8910 PM Cells;背景说明:高转移卵巢癌 Cells;传代方法:消化3-5分钟。1:2。3天内可长满。;生长特性:贴壁生长;形态特性:上皮样;相关产品有:CL-40 Cells、H-2227 Cells、NCI-H345 Cells
SHIN3 Cells;背景说明:卵巢浆液性囊腺癌;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:HO8910PM Cells、H187 Cells、PC 61 Cells
COLO.205 Cells;背景说明:该细胞系是1957年由T.U.Sample等从患有结肠癌的70岁男性白人的腹水中分离的。该病人在取腹水样品前已用5-尿嘧啶治疗4~6周。角蛋白免疫过氧化物酶染色阳性;产生CEA、IL10。;传代方法:1:2传代;生长特性:贴壁生长;形态特性:上皮样;相关产品有:PC3 Cells、RCM1 Cells、NCI-1155 Cells
Jurkat FHCRC Cells;背景说明:该细胞源自一位14岁患有T淋巴细胞白血病男性的外周血;传代方法:保持细胞密度在3—9×105cells/ml之间,1:5—1:10传代,每周换液2—3次;生长特性:悬浮生长;形态特性:圆形,单个或呈片;相关产品有:SCC-1395 Cells、KMY1022 Cells、SNK6 Cells
Jurkat FHCRC Cells;背景说明:该细胞源自一位14岁患有T淋巴细胞白血病男性的外周血;传代方法:保持细胞密度在3—9×105cells/ml之间,1:5—1:10传代,每周换液2—3次;生长特性:悬浮生长;形态特性:圆形,单个或呈片;相关产品有:SCC-1395 Cells、KMY1022 Cells、SNK6 Cells
LTEP-s Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:IEC6 Cells、CCRF-CEM/S Cells、TR 146 Cells
UO-31 Cells;背景说明:详见相关文献介绍;传代方法:1:3-1:6传代;2-3天换液1次。;生长特性:贴壁生长;形态特性:上皮样;相关产品有:Human Kidney-2 Cells、PC2 Cells、MC-3T3 Cells
H1648 Cells;背景说明:详见相关文献介绍;传代方法:1:3-1:6传代;生长特性:贴壁生长;形态特性:上皮细胞;相关产品有:HFSF Cells、Wills Eye Research Institute-Retinoblastoma-1 Cells、HBE Cells
32D/cl3 Cells;背景说明:骨髓淋巴瘤;C3H/HeJ;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:悬浮;形态特性:详见产品说明;相关产品有:H4-II-E-C3 Cells、Hs746-T Cells、EM-3 Cells
JM-Jurkat Cells;背景说明:该细胞源自一位14岁患有T淋巴细胞白血病男性的外周血;传代方法:保持细胞密度在3—9×105cells/ml之间,1:5—1:10传代,每周换液2—3次;生长特性:悬浮生长;形态特性:圆形,单个或呈片;相关产品有:PFSK-1 Cells、TE85 Cells、Hs274T Cells
K 562 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:Walker/LLC-WRC 256 Cells、RPMI2650 Cells、H-1568 Cells
OVCAR-432 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:SK-N-BE Cells、SKMEL24 Cells、Human Embryonic Kidney 293 Cells
T24[T-24]人膀胱移行细胞癌传代细胞长期复苏|送STR图谱
JM-1 Cells;背景说明:详见相关文献介绍;传代方法:换液2-3次一周;生长特性:悬浮生长 ;形态特性:淋巴母细胞样;相关产品有:RPMI No. 1846 Cells、Clone Y-1 Cells、H7721 Cells
CZ-1 Cells;背景说明:骨髓瘤;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:悬浮;形态特性:详见产品说明;相关产品有:ST Cells、EFO-27 Cells、HMC Cells
SYSUe008-A Cells(拥有STR基因鉴定图谱)
PC-10 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:NCI-HUT-69 Cells、NCIH2591 Cells、P3X63NS1 Cells
Tadarida brasiliensis 1 lung Cells;背景说明:肺;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:JCA-1 Cells、SCC15 Cells、P30/OHK Cells
P3-X63.Ag8.653 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:MonoMac 6 Cells、SU-DHL-6 Cells、BEL7405 Cells
KHYG Cells;背景说明:NK细胞淋巴瘤/白血病;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:悬浮;形态特性:详见产品说明;相关产品有:Cates 1B Cells、OVCAR8/ADR Cells、H2172 Cells
┈订┈购┈热┈线:1┈5┈8┈0┈0┈5┈7┈6┈8┈6┈7【微信同号】┈Q┈Q:3┈3┈0┈7┈2┈0┈4┈2┈7┈1;
K7M2 Cells;背景说明:骨肉瘤;肺转移;雌性;BALB/c;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:GM03569D Cells、Pro-Lec1.3C Cells、NCI-H1092 Cells
NCI.H522 Cells;背景说明:详见相关文献介绍;传代方法:1:3-1:6传代;每周换液2-3次。;生长特性:贴壁生长;形态特性:上皮样;相关产品有:PG-4 (S+L-) Cells、GM03573A Cells、NS1-1 Ag4.1 Cells
NCI-H508 Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长;形态特性:详见产品说明;相关产品有:293-H Cells、NCI660 Cells、JVM3 Cells
H358 Cells;背景说明:1981年从一位开始化疗之前的患者的肿瘤组织中分离建株。超微结构研究表明细胞质中有Clara细胞的特征结构细胞表达主要的肺表面结合蛋白SP-A的蛋白和RNA。不表达SP-B和SP-C。他们在软琼脂中的克隆形成效率为0.83%。;传代方法:消化3-5分钟。1:2。3天内可长满。;生长特性:贴壁生长;形态特性:上皮样;相关产品有:P3/NS1/1-Ag4.1 Cells、NK-92 transfected with MFG-hIL2 Cells、ACC-2 Cells
HO8910/PM Cells;背景说明:高转移卵巢癌 Cells;传代方法:消化3-5分钟。1:2。3天内可长满。;生长特性:贴壁生长;形态特性:上皮样;相关产品有:Bac12F5 Cells、U-118-MG Cells、MUS-M1 Cells
870 Cells;背景说明:儿童急性髓系白血病;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:悬浮;形态特性:详见产品说明;相关产品有:H1650 Cells、Hs600T Cells、PC-10 Cells
Becker Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长;形态特性:成纤维细胞;相关产品有:WI38 Cells、GM-637 Cells、HCC2157 Cells
CMT64 Cells;背景说明:肺腺癌;雌性;C57;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:LN382 Cells、BPH1 Cells、C57 Mouse Tumor 93 Cells
COLO320HSR Cells;背景说明:该细胞1984年建系,源自一位33岁患有大肠腺癌男性经5-fu治疗后的腹水。;传代方法:1:2传代。3天内可长满。;生长特性:半贴壁生长;形态特性:详见产品说明;相关产品有:EA.Hy926 Cells、LM3 Cells、Ly18 Cells
769P Cells;背景说明:该细胞系1975年建系,源自一位63岁白人女性的初期透明细胞腺癌组织,细胞呈圆形且边界不清,核浆比大,有微绒毛及桥粒。该细胞可在软琼脂上生长。 ;传代方法:1:4—1:12传代,2—3天换液一次;生长特性:贴壁生长;形态特性:上皮细胞样;相关产品有:HPAF-II Cells、A3 Cells、CATHa Cells
BayGenomics ES cell line CSI629 Cells(拥有STR基因鉴定图谱)
BayGenomics ES cell line ST484 Cells(拥有STR基因鉴定图谱)
CA4AF5 Cells(拥有STR基因鉴定图谱)
LP10 Cells(拥有STR基因鉴定图谱)
R7T1 Cells(拥有STR基因鉴定图谱)
RLD-9 Cells(拥有STR基因鉴定图谱)
" "PubMed=870558; DOI=10.1177/25.4.870558
Benham F.J., Cottell D.C., Franks L.M., Wilson P.D.
Alkaline phosphatase activity in human bladder tumor cell lines.
J. Histochem. Cytochem. 25:266-274(1977)
PubMed=77569; DOI=10.1111/j.1399-0039.1978.tb01259.x
Espmark J.A., Ahlqvist-Roth L., Sarne L., Persson A.
Tissue typing of cells in culture. III. HLA antigens of established human cell lines. Attempts at typing by the mixed hemadsorption technique.
Tissue Antigens 11:279-286(1978)
PubMed=571047
Fogh J.
Cultivation, characterization, and identification of human tumor cells with emphasis on kidney, testis, and bladder tumors.
Natl. Cancer Inst. Monogr. 49:5-9(1978)
PubMed=651066; DOI=10.5980/jpnjurol1928.69.1_40
Kato T., Ishikawa K., Nemoto R.
Morphologic characterization of two established cell lines, T24 and MGH-U1, derived from human bladder carcinoma.
Nihon Hinyokika Gakkai Zasshi 69:40-46(1978)
PubMed=663932; DOI=10.1620/tjem.124.33
Kato T., Ishikawa K., Nemoto R., Senoo A., Amano Y.
Morphological characterization of two established cell lines, T24 and MGH-U1, derived from human urinary bladder carcinoma.
Tohoku J. Exp. Med. 124:339-349(1978)
PubMed=6244232
Williams R.D.
Human urologic cancer cell lines.
Invest. Urol. 17:359-363(1980)
PubMed=7017212; DOI=10.1093/jnci/66.6.1003
Pollack M.S., Heagney S.D., Livingston P.O., Fogh J.
HLA-A, B, C and DR alloantigen expression on forty-six cultured human tumor cell lines.
J. Natl. Cancer Inst. 66:1003-1012(1981)
PubMed=7185004; DOI=10.2302/kjm.31.127
Tachibana M.
Studies on cellular adhesiveness in five different culture cell lines derived from carcinoma of the urinary bladder.
Keio J. Med. 31:127-148(1982)
PubMed=6220172
Dracopoli N.C., Fogh J.
Polymorphic enzyme analysis of cultured human tumor cell lines.
J. Natl. Cancer Inst. 70:469-476(1983)
PubMed=6823318; DOI=10.1038/301429a0
O'Toole C.M., Povey S., Hepburn P.J., Franks L.M.
Identity of some human bladder cancer cell lines.
Nature 301:429-430(1983)
PubMed=6826254; DOI=10.1002/ijc.2910310308
Paulie S., Hansson Y., Lundblad M.-L., Perlmann P.
Lectins as probes for identification of tumor-associated antigens on urothelial and colonic carcinoma cell lines.
Int. J. Cancer 31:297-303(1983)
PubMed=3518877; DOI=10.3109/07357908609038260
Fogh J.
Human tumor lines for cancer research.
Cancer Invest. 4:157-184(1986)
PubMed=3708594
Masters J.R.W., Hepburn P.J., Walker L., Highman W.J., Trejdosiewicz L.K., Povey S., Parkar M., Hill B.T., Riddle P.N., Franks L.M.
Tissue culture model of transitional cell carcinoma: characterization of twenty-two human urothelial cell lines.
Cancer Res. 46:3630-3636(1986)
PubMed=2607719; DOI=10.5980/jpnjurol1989.80.988
Kihara K., Kageyama Y., Sumi S., Higashi Y., Fukui I., Oshima H.
A study of intercellular communication of human transitional cell carcinoma cell lines.
Nihon Hinyokika Gakkai Zasshi 80:988-994(1989)
PubMed=7787250
Cooper M.J., Haluschak J.J., Johnson D., Schwartz S., Morrison L.J., Lippa M., Hatzivassiliou G., Tan J.
p53 mutations in bladder carcinoma cell lines.
Oncol. Res. 6:569-579(1994)
PubMed=8873383; DOI=10.1007/BF00295899
Stadler W.M., Olopade O.I.
The 9p21 region in bladder cancer cell lines: large homozygous deletion inactivate the CDKN2, CDKN2B and MTAP genes.
Urol. Res. 24:239-244(1996)
PubMed=9247707; DOI=10.1080/15216549700202901
Hatakeyama S., Gao Y.-H., Ohara-Nemoto Y., Kataoka H., Satoh M.
Expression of bone morphogenetic proteins of human neoplastic epithelial cells.
Biochem. Mol. Biol. Int. 42:497-505(1997)
PubMed=9290701; DOI=10.1002/(SICI)1098-2744(199708)19:4<243::AID-MC5>3.0.CO;2-D
Jia L.-Q., Osada M., Ishioka C., Gamo M., Ikawa S., Suzuki T., Shimodaira H., Niitani T., Kudo T., Akiyama M., Kimura N., Matsuo M., Mizusawa H., Tanaka N., Koyama H., Namba M., Kanamaru R., Kuroki T.
Screening the p53 status of human cell lines using a yeast functional assay.
Mol. Carcinog. 19:243-253(1997)
PubMed=9850064
Markl I.D.C., Jones P.A.
Presence and location of TP53 mutation determines pattern of CDKN2A/ARF pathway inactivation in bladder cancer.
Cancer Res. 58:5348-5353(1998)
DOI=10.11418/jtca1981.18.4_329
Tanabe H., Takada Y., Minegishi D., Kurematsu M., Masui T., Mizusawa H.
Cell line individualization by STR multiplex system in the cell bank found cross-contamination between ECV304 and EJ-1/T24.
Tissue Cult. Res. Commun. 18:329-338(1999)
PubMed=11416159; DOI=10.1073/pnas.121616198; PMCID=PMC35459
Masters J.R.W., Thomson J.A., Daly-Burns B., Reid Y.A., Dirks W.G., Packer P., Toji L.H., Ohno T., Tanabe H., Arlett C.F., Kelland L.R., Harrison M., Virmani A.K., Ward T.H., Ayres K.L., Debenham P.G.
Short tandem repeat profiling provides an international reference standard for human cell lines.
Proc. Natl. Acad. Sci. U.S.A. 98:8012-8017(2001)
PubMed=11921286; DOI=10.1002/gcc.10050
Williams S.V., Sibley K.D., Davies A.M., Nishiyama H., Hornigold N., Coulter J., Kennedy W.J., Skilleter A., Habuchi T., Knowles M.A.
Molecular genetic analysis of chromosome 9 candidate tumor-suppressor loci in bladder cancer cell lines.
Genes Chromosomes Cancer 34:86-96(2002)
PubMed=12068308; DOI=10.1038/nature00766
Davies H.R., Bignell G.R., Cox C., Stephens P.J., Edkins S., Clegg S., Teague J.W., Woffendin H., Garnett M.J., Bottomley W., Davis N., Dicks E., Ewing R., Floyd Y., Gray K., Hall S., Hawes R., Hughes J., Kosmidou V., Menzies A., Mould C., Parker A., Stevens C., Watt S., Hooper S., Wilson R., Jayatilake H., Gusterson B.A., Cooper C.S., Shipley J.M., Hargrave D., Pritchard-Jones K., Maitland N.J., Chenevix-Trench G., Riggins G.J., Bigner D.D., Palmieri G., Cossu A., Flanagan A.M., Nicholson A., Ho J.W.C., Leung S.Y., Yuen S.T., Weber B.L., Seigler H.F., Darrow T.L., Paterson H.F., Marais R., Marshall C.J., Wooster R., Stratton M.R., Futreal P.A.
Mutations of the BRAF gene in human cancer.
Nature 417:949-954(2002)
PubMed=15846775; DOI=10.1002/gcc.20166
Williams S.V., Adams J., Coulter J., Summersgill B.M., Shipley J.M., Knowles M.A.
Assessment by M-FISH of karyotypic complexity and cytogenetic evolution in bladder cancer in vitro.
Genes Chromosomes Cancer 43:315-328(2005)
PubMed=16885334; DOI=10.1158/0008-5472.CAN-06-1182
Lopez-Knowles E., Hernandez S., Malats N., Kogevinas M., Lloreta J., Carrato A., Tardon A., Serra C., Real F.X.
PIK3CA mutations are an early genetic alteration associated with FGFR3 mutations in superficial papillary bladder tumors.
Cancer Res. 66:7401-7404(2006)
PubMed=19105184; DOI=10.1002/pmic.200800121
Makridakis M., Gagos S., Petrolekas A., Roubelakis M.G., Bitsika V., Stravodimos K., Pavlakis K., Anagnou N.P., Coleman J.A., Vlahou A.
Chromosomal and proteome analysis of a new T24-based cell line model for aggressive bladder cancer.
Proteomics 9:287-298(2009)
PubMed=19375735; DOI=10.1016/j.juro.2009.01.108; PMCID=PMC2680455
Chiong E., Dadbin A., Harris L.D., Sabichi A.L., Grossman H.B.
The use of short tandem repeat profiling to characterize human bladder cancer cell lines.
J. Urol. 181:2737-2748(2009)
PubMed=22460905; DOI=10.1038/nature11003; PMCID=PMC3320027
Barretina J.G., Caponigro G., Stransky N., Venkatesan K., Margolin A.A., Kim S., Wilson C.J., Lehar J., Kryukov G.V., Sonkin D., Reddy A., Liu M., Murray L., Berger M.F., Monahan J.E., Morais P., Meltzer J., Korejwa A., Jane-Valbuena J., Mapa F.A., Thibault J., Bric-Furlong E., Raman P., Shipway A., Engels I.H., Cheng J., Yu G.-Y.K., Yu J.-J., Aspesi P. Jr., de Silva M., Jagtap K., Jones M.D., Wang L., Hatton C., Palescandolo E., Gupta S., Mahan S., Sougnez C., Onofrio R.C., Liefeld T., MacConaill L.E., Winckler W., Reich M., Li N.-X., Mesirov J.P., Gabriel S.B., Getz G., Ardlie K., Chan V., Myer V.E., Weber B.L., Porter J., Warmuth M., Finan P., Harris J.L., Meyerson M.L., Golub T.R., Morrissey M.P., Sellers W.R., Schlegel R., Garraway L.A.
The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity.
Nature 483:603-607(2012)
PubMed=23401075; DOI=10.1002/path.4176
Guo Y.-N., Chekaluk Y., Zhang J.-M., Du J.-Y., Gray N.S., Wu C.-L., Kwiatkowski D.J.
TSC1 involvement in bladder cancer: diverse effects and therapeutic implications.
J. Pathol. 230:17-27(2013)
PubMed=24367658; DOI=10.1371/journal.pone.0084411; PMCID=PMC3867501
Ross R.L., Burns J.E., Taylor C.F., Mellor P., Anderson D.H., Knowles M.A.
Identification of mutations in distinct regions of p85 alpha in urothelial cancer.
PLoS ONE 8:E84411-E84411(2013)
PubMed=24018021; DOI=10.1016/j.eururo.2013.08.052
Allory Y., Beukers W., Sagrera A., Flandez M., Marques M., Marquez M., van der Keur K.A., Dyrskjot L., Lurkin I., Vermeij M., Carrato A., Lloreta J., Lorente J.A., Carrillo-de-Santa-Pau E., Masius R.G., Kogevinas M., Steyerberg E.W., van Tilborg A.A.G., Abas C., Orntoft T.F., Zuiverloon T.C.M., Malats N., Zwarthoff E.C., Real F.X.
Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome.
Eur. Urol. 65:360-366(2014)
PubMed=24035680; DOI=10.1016/j.eururo.2013.08.057
Hurst C.D., Platt F.M., Knowles M.A.
Comprehensive mutation analysis of the TERT promoter in bladder cancer and detection of mutations in voided urine.
Eur. Urol. 65:367-369(2014)
PubMed=24376083; DOI=10.1002/pmic.201300452
Jeppesen D.K., Nawrocki A., Jensen S.G., Thorsen K., Whitehead B., Howard K.A., Dyrskjot L., Orntoft T.F., Larsen M.R., Ostenfeld M.S.
Quantitative proteomics of fractionated membrane and lumen exosome proteins from isogenic metastatic and nonmetastatic bladder cancer cells reveal differential expression of EMT factors.
Proteomics 14:699-712(2014)
PubMed=24459064; DOI=10.1007/s13277-013-1604-3
Pinto-Leite R., Carreira I.M., Melo J.B., Ferreira S.I., Ribeiro I.P., Ferreira J., Filipe M., Bernardo C., Arantes-Rodrigues R., Oliveira P., Santos L.
Genomic characterization of three urinary bladder cancer cell lines: understanding genomic types of urinary bladder cancer.
Tumor Biol. 35:4599-4617(2014)
PubMed=25997541; DOI=10.1186/s12864-015-1450-3; PMCID=PMC4470036
Earl J., Rico D., Carrillo-de-Santa-Pau E., Rodriguez-Santiago B., Mendez-Pertuz M., Auer H., Gomez G., Grossman H.B., Pisano D.G., Schulz W.A., Perez-Jurado L.A., Carrato A., Theodorescu D., Chanock S.J., Valencia A., Real F.X.
The UBC-40 Urothelial Bladder Cancer cell line index: a genomic resource for functional studies.
BMC Genomics 16:403.1-403.16(2015)
PubMed=26055179; DOI=10.1016/j.tranon.2015.04.002; PMCID=PMC4487788
Vallo S., Michaelis M., Rothweiler F., Bartsch G., Gust K.M., Limbart D.M., Rodel F., Wezel F., Haferkamp A., Cinatl J. Jr.
Drug-resistant urothelial cancer cell lines display diverse sensitivity profiles to potential second-line therapeutics.
Transl. Oncol. 8:210-216(2015)
PubMed=26589293; DOI=10.1186/s13073-015-0240-5; PMCID=PMC4653878
Scholtalbers J., Boegel S., Bukur T., Byl M., Goerges S., Sorn P., Loewer M., Sahin U., Castle J.C.
TCLP: an online cancer cell line catalogue integrating HLA type, predicted neo-epitopes, virus and gene expression.
Genome Med. 7:118.1-118.7(2015)
PubMed=26972028; DOI=10.1016/j.jprot.2016.03.008
Masuishi Y., Kimura Y., Arakawa N., Hirano H.
Identification of glycosylphosphatidylinositol-anchored proteins and omega-sites using TiO2-based affinity purification followed by hydrogen fluoride treatment.
J. Proteomics 139:77-83(2016)
PubMed=27141528; DOI=10.1016/j.dib.2016.04.001; PMCID=PMC4838930
Masuishi Y., Kimura Y., Arakawa N., Hirano H.
Data for identification of GPI-anchored peptides and omega-sites in cancer cell lines.
Data Brief 7:1302-1305(2016)
PubMed=27397505; DOI=10.1016/j.cell.2016.06.017; PMCID=PMC4967469
Iorio F., Knijnenburg T.A., Vis D.J., Bignell G.R., Menden M.P., Schubert M., Aben N., Goncalves E., Barthorpe S., Lightfoot H., Cokelaer T., Greninger P., van Dyk E., Chang H., de Silva H., Heyn H., Deng X.-M., Egan R.K., Liu Q.-S., Miroo T., Mitropoulos X., Richardson L., Wang J.-H., Zhang T.-H., Moran S., Sayols S., Soleimani M., Tamborero D., Lopez-Bigas N., Ross-Macdonald P., Esteller M., Gray N.S., Haber D.A., Stratton M.R., Benes C.H., Wessels L.F.A., Saez-Rodriguez J., McDermott U., Garnett M.J.
A landscape of pharmacogenomic interactions in cancer.
Cell 166:740-754(2016)
PubMed=27270441; DOI=10.1038/onc.2016.172; PMCID=PMC5140783
Nickerson M.L., Witte N., McGee Im K., Turan S., Owens C.R., Misner K., Tsang S.X., Cai Z.-M., Wu S., Dean M., Costello J.C., Theodorescu D.
Molecular analysis of urothelial cancer cell lines for modeling tumor biology and drug response.
Oncogene 36:35-46(2017)
PubMed=28196595; DOI=10.1016/j.ccell.2017.01.005; PMCID=PMC5501076
Li J., Zhao W., Akbani R., Liu W.-B., Ju Z.-L., Ling S.-Y., Vellano C.P., Roebuck P., Yu Q.-H., Eterovic A.K., Byers L.A., Davies M.A., Deng W.-L., Gopal Y.N.V., Chen G., von Euw E.M., Slamon D.J., Conklin D., Heymach J.V., Gazdar A.F., Minna J.D., Myers J.N., Lu Y.-L., Mills G.B., Liang H.
Characterization of human cancer cell lines by reverse-phase protein arrays.
Cancer Cell 31:225-239(2017)
PubMed=29732388; DOI=10.3233/BLC-180167; PMCID=PMC5929350
Zuiverloon T.C.M., de Jong F.C., Costello J.C., Theodorescu D.
Systematic review: characteristics and preclinical uses of bladder cancer cell lines.
Bladder Cancer 4:169-183(2018)
PubMed=30193179; DOI=10.1016/j.jmbbm.2018.08.036
Raczkowska J., Prauzner-Bechcicki S.
Discrimination between HCV29 and T24 by controlled proliferation of cells co-cultured on substrates with different elasticity.
J. Mech. Behav. Biomed. Mater. 88:217-222(2018)
CLPUB00543
Min Q., Cao S.-N., Gan Y.-P., Song T.-S., Liu F.-X., Ning Z.-F.
Bladder cancer chemotherapy resistant cell harbors stem-like characteristics construction and characterization of T24 resistance strain of bladder cancer.
Clin. Oncol. J. 1:1004.09-1004.12(2019)
PubMed=30894373; DOI=10.1158/0008-5472.CAN-18-2747; PMCID=PMC6445675
Dutil J., Chen Z.-H., Monteiro A.N.A., Teer J.K., Eschrich S.A.
An interactive resource to probe genetic diversity and estimated ancestry in cancer cell lines.
Cancer Res. 79:1263-1273(2019)"
传代方法:1:2-1:4(首次传代建议1:2)
生长特性:贴壁生长
换液频率:每周2-3次
背景资料:该细胞源自一位81岁白人女性患者的膀胱移行细胞癌组织;来源于移行细胞癌病人的白血病和血浆对T24和相关细胞株有细胞毒性;倍增时间为19小时;含ras(H-ras)癌基因,表达肿瘤特有抗原。
关于细胞株是否都能一直传代,答案是否定的。细胞系分为有限细胞系与连续细胞系两类。有限细胞系就像一位有着既定行程的行者,其传代之旅存在明确的终点。以正常的人体肝细胞系为例,在体外培养时,它大约能传代20-30次。随着传代次数的递增,细胞内部仿佛一台精密仪器的零件逐渐磨损老化。端粒酶活性降低,端粒不断缩短,染色体结构开始不稳定,基因表达也出现异常。同时,细胞的代谢速率减缓,对营养物质的摄取和利用效率大打折扣,有害物质的积累却日益增多,最终导致细胞停止分裂,走向生命的尽头。而连续细胞系则像是拥有无限活力的长跑健将,具有较强的传代能力。如Hela细胞系,自1951年从Henrietta Lacks女士的宫颈癌组织中分离出来后,便在全球生物实验室中“大放异彩”,至今已传代无数次。它能持续分裂得益于其特殊的遗传变异,使其染色体端粒能够维持稳定长度,并且细胞内的一些关键信号通路持续激活,促进细胞增殖。但这并不意味着它的传代毫无风险与限制。在漫长的传代过程中,它可能会发生新的基因突变、染色体易位等变异事件,从而改变细胞的生物学特性,如细胞形态、生长速度、对药物的敏感性等。
┈订┈购┈热┈线:1┈5┈8┈0┈0┈5┈7┈6┈8┈6┈7【微信同号】┈Q┈Q:3┈3┈0┈7┈2┈0┈4┈2┈7┈1;
GM07483 Cells(拥有STR基因鉴定图谱)
NCI-H1650 Cells;背景说明:该细胞是从一名27岁白人男性(10年烟龄)支气管肺泡癌患者的胸腔积液中分离得到的。;传代方法:1:4-1:6传代;2-3天换液1次。;生长特性:贴壁生长;形态特性:上皮细胞样;相关产品有:P30/0HK Cells、Normal fibroblast-10 Cells、SKRC-42 Cells
CL-34 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:MDA134 Cells、IOSE-29 Cells、Hep 3B Cells
T24[T-24]人膀胱移行细胞癌传代细胞长期复苏|送STR图谱
产品包装形式:复苏细胞:T25培养瓶(一瓶)或冻存细胞:1ml冻存管(两支)
来源说明:细胞主要来源ATCC、DSMZ等细胞库
悬浮细胞不容易转染:悬浮细胞是指细胞生长不依赖支持物表面,在培养中呈悬浮状态生长,如淋巴细胞。在实验室经常会遇到悬浮细胞的转染,其和贴壁细胞转染还是有很大不同的。目前大多数实验室用的是脂质体类的转染试剂,脂质体转染是基于电荷吸引原理,先形成脂质体-DNA复合物,散布在细胞周围,然后通过细胞的内吞作用,将目的基因导入细胞内,而脂质体复合物与贴壁细胞的接触机会比悬浮细胞GAO出很多倍,所以,脂质体转染时悬浮细胞的转染效率要明显低于贴壁细胞。其次,脂质体试剂的毒性较大,这就使得悬浮细胞的转染更为困难了。另外,悬浮细胞不易培养,易死亡,也给细胞转染造成了一定的困难。为了提GAO悬浮细胞的转染效率,可以使用非脂质体的转染试剂,如纳米材料的。也可以使用电转染方法,针对悬浮细胞等难转染细胞还是挺不错的,电击对细胞有一定的损伤,ZuiHAO选用具有细胞膜修复功能的电转染试剂,可以将电击对细胞的伤害降到Zui低,悬浮细胞不易转染,选对试剂及良HAO的细胞培养环境才是关键。
物种来源:Human\Mouse\Rat\Others
293 H Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:RGC5 Cells、NCI-H2291 Cells、H-125 Cells
GM07404D Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长 ;形态特性:详见产品说明;相关产品有:H1838 Cells、NS20Y Cells、KP2 Cells
RSC-364 Cells;背景说明:滑膜 Cells;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:NCI-H-128 Cells、KMBC Cells、MES-SA Cells
T24[T-24]人膀胱移行细胞癌传代细胞长期复苏|送STR图谱
形态特性:上皮细胞样
┈订┈购┈热┈线:1┈5┈8┈0┈0┈5┈7┈6┈8┈6┈7【微信同号】┈Q┈Q:3┈3┈0┈7┈2┈0┈4┈2┈7┈1;
细胞根据其形态和功能可以分为多种类型,其中上皮细胞、成纤维细胞和淋巴母细胞是常见的三种类型。成纤维细胞,或称纤维母细胞,是一种存在于蜂窝组织或纤维结缔组织中的梭形细胞,能够分泌构成细胞外基质(ECM)的结构蛋白。这类细胞最初由德国病理学家鲁道夫·菲尔绍与法国解剖学家马蒂亚斯·杜瓦尔于19世纪中叶描述。成纤维细胞特异性标志物包括FAP(成纤维细胞激活蛋白)、α-SMA(α-平滑肌肌动蛋白)、骨膜蛋白、PDGFRα(血小板衍生生长因子受体α)、胶原蛋白、波形蛋白、纤连蛋白和胸腺细胞抗原1等。成纤维细胞在形态上是扁平的纺锤形细胞,缺乏基底膜,细胞核呈圆形拉长,被巨大的内质网包围。在培养中,它们表现出不同的形态学和生化特征,这取决于其起源位置、分化状态和培养条件。
CHO-K1 Cells;背景说明:1957年,PuckTT从成年中国仓鼠卵巢的活检组织建立了CHO细胞,CHO-K1是CHO的一个亚克隆。CHO-K1的生长需要脯酸。;传代方法:1:2传代;生长特性:贴壁生长;形态特性:上皮样;相关产品有:V 79 Cells、MCF 7 Cells、NCI-HUT-69 Cells
NCI-H378 Cells;背景说明:小细胞肺癌;胸腔积液转移;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:WRL-68 Cells、RC-4B Cells、KHYG Cells
FL-83B Cells;背景说明:肝;自发永生;C57BL/6J;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:SW954 Cells、Hi-5 Cells、MDA175 Cells
JB6 Cl 30 Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长;形态特性:上皮样;相关产品有:MD Anderson-Metastatic Breast-435 Cells、HEY-A8 Cells、NCIH1184 Cells
L615 Cells;背景说明:白血病;615小鼠;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:悬浮;形态特性:详见产品说明;相关产品有:GM02132C Cells、KOSC-2 Cells、Earles's cells Cells
U118-MG Cells;背景说明:注意: 据报道来自不同个体的胶质母细胞瘤细胞株U-118 MG (HTB-15) 和 U-138 MG (HTB-16)有着一致的VNTR和相近的STR模式。 U-118 MG 和 U-138 MG细胞遗传学上很相似并有至少六个衍生标记染色体。 这是1966年至1969年间J. Ponten和同事从恶性神经胶质瘤中构建的细胞株中的一株(其它包括ATCC HTB-14和 ATCC HTB-16 and ATCC HTB-17)。 1987年用BM-Cycline培养6周去除了支原体污染。 ;传代方法: 消化3-5分钟。1:2传代。3天内可长满。;生长特性:贴壁生长;形态特性:混合型;相关产品有:SV-HUC Cells、brain-derived Endothelial cells.3 Cells、GH 3 Cells
BHP 10-3 Cells;背景说明:甲状腺乳头状癌;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:W133 Cells、Jurkat clone A3 Cells、RPMI 1788 Cells
NCI-SNU-761 Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:293 Cells、KM12 Cells、SNU-182 Cells
HPDE Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长 ;形态特性:详见产品说明;相关产品有:PC2 Cells、CTSC-3 Cells、P3X63 AG 8.653 Cells
FHCRC-11 Cells;背景说明:该细胞源自一位14岁患有T淋巴细胞白血病男性的外周血;传代方法:保持细胞密度在3—9×105cells/ml之间,1:5—1:10传代,每周换液2—3次;生长特性:悬浮生长;形态特性:圆形,单个或呈片;相关产品有:Metastatic Variant-522 Cells、SupT1 Cells、NCI-H2227 Cells
CCLP1 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:HS0578T Cells、P3X63-Ag8.653 Cells、F442-A Cells
OCM-1 Cells;背景说明:葡萄膜黑色素瘤;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:BV-173 Cells、GM03320D Cells、Mv1.Lu Cells
alpha-TC1-6 Cells;背景说明:胰岛素瘤;a细胞;C57BL/6xDBA/2;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:SW-527 Cells、CTV-1 Cells、SLK Cells
HBEpiC Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:RWPE-2 Cells、SKO3 Cells、GM03571 Cells
AD293 Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长;形态特性:详见产品说明;相关产品有:T-T Cells、MV4:11 Cells、ID8/MOSEC Cells
QGY 7701 Cells;背景说明:详见相关文献介绍;传代方法:消化3-5分钟,1:2,3天内可长满;生长特性:贴壁生长;形态特性:上皮样;相关产品有:NCI H23 Cells、HOPC Cells、MS1 Cells
H596 Cells;背景说明:详见相关文献介绍;传代方法:1:4-1:8传代;每周换液2-3次。;生长特性:贴壁生长;形态特性:上皮样;相关产品有:GA-10(Clone 4) Cells、CCD 19Lu Cells、Dakiki Cells
Abcam A-549 CBL KO Cells(拥有STR基因鉴定图谱)
Abcam Raji TPP2 KO Cells(拥有STR基因鉴定图谱)
BayGenomics ES cell line BGC386 Cells(拥有STR基因鉴定图谱)
BayGenomics ES cell line RRS151 Cells(拥有STR基因鉴定图谱)
BayGenomics ES cell line YTA400 Cells(拥有STR基因鉴定图谱)
Chem-9 GLP1R Cells(拥有STR基因鉴定图谱)
DA01890 Cells(拥有STR基因鉴定图谱)
F15553 Cells(拥有STR基因鉴定图谱)
PNT1-a Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:DHL4 Cells、GC-1 Cells、MSTO-211 Cells
KM12-SM Cells;背景说明:结肠癌;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:143B TK- Cells、Potorous tridactylus Kidney 2 Cells、HLFa Cells
T24[T-24]人膀胱移行细胞癌传代细胞长期复苏|送STR图谱
MSTO211H Cells;背景说明:MSTO-211H细胞株是1985年从一位肺二相间皮瘤患者的胸水中建株的。这个病人接受过多种药物联合前期化疗。MSTO-211H细胞具有高亲和力的EGF结合位点,并表达神经元特异性烯醇酶(NSE)及人绒毛膜促性腺激素(HCG)的α与β亚基。未检测到左旋多巴胺脱羧酶(DDC),邦巴辛与神经tensin。细胞过表达c-myc原癌基因,并没有观察到基因重排或扩增。V-src,v-abl,v-erbB,c-raf1,Ha-ras,Ki-ras,和N-ras的表达呈阳性。未检测到N-m;传代方法:消化3-5分钟。1:2。3天内可长满。;生长特性:贴壁生长;形态特性:成纤维细胞样;相关产品有:NFS-60 Cells、SUM-159PT Cells、CATH-a Cells
VMM39 Cells;背景说明:黑色素瘤;神经节转移;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:A498 Cells、SK-ML2 Cells、KCL22 Cells
HG2855 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:P116 Cells、H-735 Cells、MLE-12 Cells
SW 480 Cells;背景说明:SW480源自一位51岁白人男性患者的原位直肠腺癌,而SW620源自同一病人一年后的淋巴结转移灶。该细胞CSAp和直肠抗原3阴性;角蛋白阳性;p53基因第273位密码子的G→A突变引起Arg→His替代,309位密码子的C→T突变导致Pro→Ser替代;细胞p53蛋白表达水平升高;癌基因c-myc、K-ras、H-ras、N-ras、myb、sis和fos的表达呈阳性;未检测到癌基因N-myc的表达;不表达Matrilysin(一种与肿瘤侵袭相关的金属蛋白酶)。;传代方法:1:2传代,1-2天换液一次;生长特性:贴壁生长;形态特性:上皮样;相关产品有:Balb/c 3T3 Cells、CAL120 Cells、NCI/ADR-RES Cells
VP 229 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:COLO-680N Cells、BTI-Tn-5B1-4 Cells、UM-UC3 Cells
NCI-H322 Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长;形态特性:详见产品说明;相关产品有:CEM-T4 Cells、MCA205 Cells、BT 20 Cells
AZc6#22 Cells(拥有STR基因鉴定图谱)
TSCC1 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:SW-756 Cells、M4e Cells、HuP-T4 Cells
HC11 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:MM6 Cells、HEC-251 Cells、NUGC4 Cells
┈订┈购┈热┈线:1┈5┈8┈0┈0┈5┈7┈6┈8┈6┈7【微信同号】┈Q┈Q:3┈3┈0┈7┈2┈0┈4┈2┈7┈1;
Panc 08.13 Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长;形态特性:上皮样;相关产品有:AZ 521 Cells、HKF Cells、HSAS1 Cells
ECC1 Cells;背景说明:内膜腺癌;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:MC3T3-E1 Subclone 24 Cells、GM-3573 Cells、DKMG Cells
GA-10 clone 4 Cells;背景说明:详见相关文献介绍;传代方法:每2-3天换液;生长特性:悬浮生长 ;形态特性:淋巴母细胞样;相关产品有:P388.D1 Cells、Hs 636 T Cells、SNU-869 Cells
HUT 125 Cells;背景说明:腺鳞状肺癌;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:LTEP a2 Cells、TPC1 Cells、SW260 Cells
HUT 125 Cells;背景说明:腺鳞状肺癌;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:LTEP a2 Cells、TPC1 Cells、SW260 Cells
High5 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:C2BBe 1 Cells、Kit225 K6 Cells、BrCL15 Cells
GT38 Cells(拥有STR基因鉴定图谱)
HAP1 PRKAG1 (-) 1 Cells(拥有STR基因鉴定图谱)
A2008 Cells;背景说明:宫颈鳞癌;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:OsACL Cells、AU-Mel Cells、H-35 Reuber Cells
H-2291 Cells;背景说明:详见相关文献介绍;传代方法:1:3-1:4传代;每周换液2-3次。;生长特性:贴壁生长;形态特性:上皮细胞样;相关产品有:SuDHL 4 Cells、BCaP-37 Cells、HeLa/DDP Cells
KGN Cells;背景说明:该细胞株是颗粒细胞瘤。细胞在加入人体绒膜促性腺激素后可能产生孕酮。细胞生长缓慢。;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:上皮细胞样;相关产品有:SO-RB50 Cells、HS688AT Cells、SKCO1 Cells
J774 A1 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:Hs 895.T Cells、C-26 Cells、OCIAML3 Cells
LNCaP-Clone-FGC Cells;背景说明:人前列腺癌细胞LNCaP克隆FGC是从一位50岁白人男性(血型B+)的左锁骨淋巴结针刺活检中分离,该患者经确诊为前列腺癌转移。 这株细胞对5-α-二睾酮(生长调节子和酸性脂酶产物)有响应。这株细胞并不形成一致的单层,而是形成集落,在传代时可以用滴管反复吹吸打碎。它们仅仅轻轻地吸附在基底上,不形成汇合,很快使培养基变酸。生长很慢。传代后48小时内不应扰动。当培养瓶封包后,多数细胞从培养瓶底分离,悬浮在培养基中。收到后,在通常培养单层细胞的条件下培养24到48小时,以合细胞再贴壁。;传代方法:消化3-5分钟。1:2。3天内可长满。;生长特性:贴壁生长;形态特性:上皮细胞;相关产品有:H1954 Cells、3T6 Swiss Albino Cells、Tj-905 Cells
MDST8 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:NCIH2286 Cells、A-704 Cells、MBdSMC Cells
Hep G2-Luc Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:SUDHL-5 Cells、A673 Cells、Ls-174-T Cells
Centre Antoine Lacassagne-62 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:MESSA Cells、IHC-ST1 Cells、CEM-CCRF (CAMR) Cells
HUES 37 Cells(拥有STR基因鉴定图谱)
KYSE-360 Cells(拥有STR基因鉴定图谱)
MS-LG Cells(拥有STR基因鉴定图谱)
OCUCh-LM1 Cells(拥有STR基因鉴定图谱)
RMO1 Cells(拥有STR基因鉴定图谱)
Ubigene HCT 116 TNFRSF13C KO Cells(拥有STR基因鉴定图谱)
WAe025-A-1 Cells(拥有STR基因鉴定图谱)
HeLa S3 AGO2KO Cells(拥有STR基因鉴定图谱)
293A Cells;背景说明:胚肾;腺病毒包装;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:WC00044 Cells、A-2058 Cells、4175 Cells
HO-8910 PM Cells;背景说明:高转移卵巢癌 Cells;传代方法:消化3-5分钟。1:2。3天内可长满。;生长特性:贴壁生长;形态特性:上皮样;相关产品有:CL-40 Cells、H-2227 Cells、NCI-H345 Cells
SHIN3 Cells;背景说明:卵巢浆液性囊腺癌;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:HO8910PM Cells、H187 Cells、PC 61 Cells
COLO.205 Cells;背景说明:该细胞系是1957年由T.U.Sample等从患有结肠癌的70岁男性白人的腹水中分离的。该病人在取腹水样品前已用5-尿嘧啶治疗4~6周。角蛋白免疫过氧化物酶染色阳性;产生CEA、IL10。;传代方法:1:2传代;生长特性:贴壁生长;形态特性:上皮样;相关产品有:PC3 Cells、RCM1 Cells、NCI-1155 Cells
Jurkat FHCRC Cells;背景说明:该细胞源自一位14岁患有T淋巴细胞白血病男性的外周血;传代方法:保持细胞密度在3—9×105cells/ml之间,1:5—1:10传代,每周换液2—3次;生长特性:悬浮生长;形态特性:圆形,单个或呈片;相关产品有:SCC-1395 Cells、KMY1022 Cells、SNK6 Cells
Jurkat FHCRC Cells;背景说明:该细胞源自一位14岁患有T淋巴细胞白血病男性的外周血;传代方法:保持细胞密度在3—9×105cells/ml之间,1:5—1:10传代,每周换液2—3次;生长特性:悬浮生长;形态特性:圆形,单个或呈片;相关产品有:SCC-1395 Cells、KMY1022 Cells、SNK6 Cells
LTEP-s Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:IEC6 Cells、CCRF-CEM/S Cells、TR 146 Cells
UO-31 Cells;背景说明:详见相关文献介绍;传代方法:1:3-1:6传代;2-3天换液1次。;生长特性:贴壁生长;形态特性:上皮样;相关产品有:Human Kidney-2 Cells、PC2 Cells、MC-3T3 Cells
H1648 Cells;背景说明:详见相关文献介绍;传代方法:1:3-1:6传代;生长特性:贴壁生长;形态特性:上皮细胞;相关产品有:HFSF Cells、Wills Eye Research Institute-Retinoblastoma-1 Cells、HBE Cells
32D/cl3 Cells;背景说明:骨髓淋巴瘤;C3H/HeJ;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:悬浮;形态特性:详见产品说明;相关产品有:H4-II-E-C3 Cells、Hs746-T Cells、EM-3 Cells
JM-Jurkat Cells;背景说明:该细胞源自一位14岁患有T淋巴细胞白血病男性的外周血;传代方法:保持细胞密度在3—9×105cells/ml之间,1:5—1:10传代,每周换液2—3次;生长特性:悬浮生长;形态特性:圆形,单个或呈片;相关产品有:PFSK-1 Cells、TE85 Cells、Hs274T Cells
K 562 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:Walker/LLC-WRC 256 Cells、RPMI2650 Cells、H-1568 Cells
OVCAR-432 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:SK-N-BE Cells、SKMEL24 Cells、Human Embryonic Kidney 293 Cells
T24[T-24]人膀胱移行细胞癌传代细胞长期复苏|送STR图谱
JM-1 Cells;背景说明:详见相关文献介绍;传代方法:换液2-3次一周;生长特性:悬浮生长 ;形态特性:淋巴母细胞样;相关产品有:RPMI No. 1846 Cells、Clone Y-1 Cells、H7721 Cells
CZ-1 Cells;背景说明:骨髓瘤;男性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:悬浮;形态特性:详见产品说明;相关产品有:ST Cells、EFO-27 Cells、HMC Cells
SYSUe008-A Cells(拥有STR基因鉴定图谱)
PC-10 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:NCI-HUT-69 Cells、NCIH2591 Cells、P3X63NS1 Cells
Tadarida brasiliensis 1 lung Cells;背景说明:肺;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:JCA-1 Cells、SCC15 Cells、P30/OHK Cells
P3-X63.Ag8.653 Cells;背景说明:详见相关文献介绍;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁或悬浮,详见产品说明部分;形态特性:详见产品说明;相关产品有:MonoMac 6 Cells、SU-DHL-6 Cells、BEL7405 Cells
KHYG Cells;背景说明:NK细胞淋巴瘤/白血病;女性;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:悬浮;形态特性:详见产品说明;相关产品有:Cates 1B Cells、OVCAR8/ADR Cells、H2172 Cells
┈订┈购┈热┈线:1┈5┈8┈0┈0┈5┈7┈6┈8┈6┈7【微信同号】┈Q┈Q:3┈3┈0┈7┈2┈0┈4┈2┈7┈1;
K7M2 Cells;背景说明:骨肉瘤;肺转移;雌性;BALB/c;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:GM03569D Cells、Pro-Lec1.3C Cells、NCI-H1092 Cells
NCI.H522 Cells;背景说明:详见相关文献介绍;传代方法:1:3-1:6传代;每周换液2-3次。;生长特性:贴壁生长;形态特性:上皮样;相关产品有:PG-4 (S+L-) Cells、GM03573A Cells、NS1-1 Ag4.1 Cells
NCI-H508 Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长;形态特性:详见产品说明;相关产品有:293-H Cells、NCI660 Cells、JVM3 Cells
H358 Cells;背景说明:1981年从一位开始化疗之前的患者的肿瘤组织中分离建株。超微结构研究表明细胞质中有Clara细胞的特征结构细胞表达主要的肺表面结合蛋白SP-A的蛋白和RNA。不表达SP-B和SP-C。他们在软琼脂中的克隆形成效率为0.83%。;传代方法:消化3-5分钟。1:2。3天内可长满。;生长特性:贴壁生长;形态特性:上皮样;相关产品有:P3/NS1/1-Ag4.1 Cells、NK-92 transfected with MFG-hIL2 Cells、ACC-2 Cells
HO8910/PM Cells;背景说明:高转移卵巢癌 Cells;传代方法:消化3-5分钟。1:2。3天内可长满。;生长特性:贴壁生长;形态特性:上皮样;相关产品有:Bac12F5 Cells、U-118-MG Cells、MUS-M1 Cells
870 Cells;背景说明:儿童急性髓系白血病;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:悬浮;形态特性:详见产品说明;相关产品有:H1650 Cells、Hs600T Cells、PC-10 Cells
Becker Cells;背景说明:详见相关文献介绍;传代方法:1:2传代;生长特性:贴壁生长;形态特性:成纤维细胞;相关产品有:WI38 Cells、GM-637 Cells、HCC2157 Cells
CMT64 Cells;背景说明:肺腺癌;雌性;C57;传代方法:1:2-1:3传代;每周换液2-3次。;生长特性:贴壁;形态特性:详见产品说明;相关产品有:LN382 Cells、BPH1 Cells、C57 Mouse Tumor 93 Cells
COLO320HSR Cells;背景说明:该细胞1984年建系,源自一位33岁患有大肠腺癌男性经5-fu治疗后的腹水。;传代方法:1:2传代。3天内可长满。;生长特性:半贴壁生长;形态特性:详见产品说明;相关产品有:EA.Hy926 Cells、LM3 Cells、Ly18 Cells
769P Cells;背景说明:该细胞系1975年建系,源自一位63岁白人女性的初期透明细胞腺癌组织,细胞呈圆形且边界不清,核浆比大,有微绒毛及桥粒。该细胞可在软琼脂上生长。 ;传代方法:1:4—1:12传代,2—3天换液一次;生长特性:贴壁生长;形态特性:上皮细胞样;相关产品有:HPAF-II Cells、A3 Cells、CATHa Cells
BayGenomics ES cell line CSI629 Cells(拥有STR基因鉴定图谱)
BayGenomics ES cell line ST484 Cells(拥有STR基因鉴定图谱)
CA4AF5 Cells(拥有STR基因鉴定图谱)
LP10 Cells(拥有STR基因鉴定图谱)
R7T1 Cells(拥有STR基因鉴定图谱)
RLD-9 Cells(拥有STR基因鉴定图谱)
" "PubMed=870558; DOI=10.1177/25.4.870558
Benham F.J., Cottell D.C., Franks L.M., Wilson P.D.
Alkaline phosphatase activity in human bladder tumor cell lines.
J. Histochem. Cytochem. 25:266-274(1977)
PubMed=77569; DOI=10.1111/j.1399-0039.1978.tb01259.x
Espmark J.A., Ahlqvist-Roth L., Sarne L., Persson A.
Tissue typing of cells in culture. III. HLA antigens of established human cell lines. Attempts at typing by the mixed hemadsorption technique.
Tissue Antigens 11:279-286(1978)
PubMed=571047
Fogh J.
Cultivation, characterization, and identification of human tumor cells with emphasis on kidney, testis, and bladder tumors.
Natl. Cancer Inst. Monogr. 49:5-9(1978)
PubMed=651066; DOI=10.5980/jpnjurol1928.69.1_40
Kato T., Ishikawa K., Nemoto R.
Morphologic characterization of two established cell lines, T24 and MGH-U1, derived from human bladder carcinoma.
Nihon Hinyokika Gakkai Zasshi 69:40-46(1978)
PubMed=663932; DOI=10.1620/tjem.124.33
Kato T., Ishikawa K., Nemoto R., Senoo A., Amano Y.
Morphological characterization of two established cell lines, T24 and MGH-U1, derived from human urinary bladder carcinoma.
Tohoku J. Exp. Med. 124:339-349(1978)
PubMed=6244232
Williams R.D.
Human urologic cancer cell lines.
Invest. Urol. 17:359-363(1980)
PubMed=7017212; DOI=10.1093/jnci/66.6.1003
Pollack M.S., Heagney S.D., Livingston P.O., Fogh J.
HLA-A, B, C and DR alloantigen expression on forty-six cultured human tumor cell lines.
J. Natl. Cancer Inst. 66:1003-1012(1981)
PubMed=7185004; DOI=10.2302/kjm.31.127
Tachibana M.
Studies on cellular adhesiveness in five different culture cell lines derived from carcinoma of the urinary bladder.
Keio J. Med. 31:127-148(1982)
PubMed=6220172
Dracopoli N.C., Fogh J.
Polymorphic enzyme analysis of cultured human tumor cell lines.
J. Natl. Cancer Inst. 70:469-476(1983)
PubMed=6823318; DOI=10.1038/301429a0
O'Toole C.M., Povey S., Hepburn P.J., Franks L.M.
Identity of some human bladder cancer cell lines.
Nature 301:429-430(1983)
PubMed=6826254; DOI=10.1002/ijc.2910310308
Paulie S., Hansson Y., Lundblad M.-L., Perlmann P.
Lectins as probes for identification of tumor-associated antigens on urothelial and colonic carcinoma cell lines.
Int. J. Cancer 31:297-303(1983)
PubMed=3518877; DOI=10.3109/07357908609038260
Fogh J.
Human tumor lines for cancer research.
Cancer Invest. 4:157-184(1986)
PubMed=3708594
Masters J.R.W., Hepburn P.J., Walker L., Highman W.J., Trejdosiewicz L.K., Povey S., Parkar M., Hill B.T., Riddle P.N., Franks L.M.
Tissue culture model of transitional cell carcinoma: characterization of twenty-two human urothelial cell lines.
Cancer Res. 46:3630-3636(1986)
PubMed=2607719; DOI=10.5980/jpnjurol1989.80.988
Kihara K., Kageyama Y., Sumi S., Higashi Y., Fukui I., Oshima H.
A study of intercellular communication of human transitional cell carcinoma cell lines.
Nihon Hinyokika Gakkai Zasshi 80:988-994(1989)
PubMed=7787250
Cooper M.J., Haluschak J.J., Johnson D., Schwartz S., Morrison L.J., Lippa M., Hatzivassiliou G., Tan J.
p53 mutations in bladder carcinoma cell lines.
Oncol. Res. 6:569-579(1994)
PubMed=8873383; DOI=10.1007/BF00295899
Stadler W.M., Olopade O.I.
The 9p21 region in bladder cancer cell lines: large homozygous deletion inactivate the CDKN2, CDKN2B and MTAP genes.
Urol. Res. 24:239-244(1996)
PubMed=9247707; DOI=10.1080/15216549700202901
Hatakeyama S., Gao Y.-H., Ohara-Nemoto Y., Kataoka H., Satoh M.
Expression of bone morphogenetic proteins of human neoplastic epithelial cells.
Biochem. Mol. Biol. Int. 42:497-505(1997)
PubMed=9290701; DOI=10.1002/(SICI)1098-2744(199708)19:4<243::AID-MC5>3.0.CO;2-D
Jia L.-Q., Osada M., Ishioka C., Gamo M., Ikawa S., Suzuki T., Shimodaira H., Niitani T., Kudo T., Akiyama M., Kimura N., Matsuo M., Mizusawa H., Tanaka N., Koyama H., Namba M., Kanamaru R., Kuroki T.
Screening the p53 status of human cell lines using a yeast functional assay.
Mol. Carcinog. 19:243-253(1997)
PubMed=9850064
Markl I.D.C., Jones P.A.
Presence and location of TP53 mutation determines pattern of CDKN2A/ARF pathway inactivation in bladder cancer.
Cancer Res. 58:5348-5353(1998)
DOI=10.11418/jtca1981.18.4_329
Tanabe H., Takada Y., Minegishi D., Kurematsu M., Masui T., Mizusawa H.
Cell line individualization by STR multiplex system in the cell bank found cross-contamination between ECV304 and EJ-1/T24.
Tissue Cult. Res. Commun. 18:329-338(1999)
PubMed=11416159; DOI=10.1073/pnas.121616198; PMCID=PMC35459
Masters J.R.W., Thomson J.A., Daly-Burns B., Reid Y.A., Dirks W.G., Packer P., Toji L.H., Ohno T., Tanabe H., Arlett C.F., Kelland L.R., Harrison M., Virmani A.K., Ward T.H., Ayres K.L., Debenham P.G.
Short tandem repeat profiling provides an international reference standard for human cell lines.
Proc. Natl. Acad. Sci. U.S.A. 98:8012-8017(2001)
PubMed=11921286; DOI=10.1002/gcc.10050
Williams S.V., Sibley K.D., Davies A.M., Nishiyama H., Hornigold N., Coulter J., Kennedy W.J., Skilleter A., Habuchi T., Knowles M.A.
Molecular genetic analysis of chromosome 9 candidate tumor-suppressor loci in bladder cancer cell lines.
Genes Chromosomes Cancer 34:86-96(2002)
PubMed=12068308; DOI=10.1038/nature00766
Davies H.R., Bignell G.R., Cox C., Stephens P.J., Edkins S., Clegg S., Teague J.W., Woffendin H., Garnett M.J., Bottomley W., Davis N., Dicks E., Ewing R., Floyd Y., Gray K., Hall S., Hawes R., Hughes J., Kosmidou V., Menzies A., Mould C., Parker A., Stevens C., Watt S., Hooper S., Wilson R., Jayatilake H., Gusterson B.A., Cooper C.S., Shipley J.M., Hargrave D., Pritchard-Jones K., Maitland N.J., Chenevix-Trench G., Riggins G.J., Bigner D.D., Palmieri G., Cossu A., Flanagan A.M., Nicholson A., Ho J.W.C., Leung S.Y., Yuen S.T., Weber B.L., Seigler H.F., Darrow T.L., Paterson H.F., Marais R., Marshall C.J., Wooster R., Stratton M.R., Futreal P.A.
Mutations of the BRAF gene in human cancer.
Nature 417:949-954(2002)
PubMed=15846775; DOI=10.1002/gcc.20166
Williams S.V., Adams J., Coulter J., Summersgill B.M., Shipley J.M., Knowles M.A.
Assessment by M-FISH of karyotypic complexity and cytogenetic evolution in bladder cancer in vitro.
Genes Chromosomes Cancer 43:315-328(2005)
PubMed=16885334; DOI=10.1158/0008-5472.CAN-06-1182
Lopez-Knowles E., Hernandez S., Malats N., Kogevinas M., Lloreta J., Carrato A., Tardon A., Serra C., Real F.X.
PIK3CA mutations are an early genetic alteration associated with FGFR3 mutations in superficial papillary bladder tumors.
Cancer Res. 66:7401-7404(2006)
PubMed=19105184; DOI=10.1002/pmic.200800121
Makridakis M., Gagos S., Petrolekas A., Roubelakis M.G., Bitsika V., Stravodimos K., Pavlakis K., Anagnou N.P., Coleman J.A., Vlahou A.
Chromosomal and proteome analysis of a new T24-based cell line model for aggressive bladder cancer.
Proteomics 9:287-298(2009)
PubMed=19375735; DOI=10.1016/j.juro.2009.01.108; PMCID=PMC2680455
Chiong E., Dadbin A., Harris L.D., Sabichi A.L., Grossman H.B.
The use of short tandem repeat profiling to characterize human bladder cancer cell lines.
J. Urol. 181:2737-2748(2009)
PubMed=22460905; DOI=10.1038/nature11003; PMCID=PMC3320027
Barretina J.G., Caponigro G., Stransky N., Venkatesan K., Margolin A.A., Kim S., Wilson C.J., Lehar J., Kryukov G.V., Sonkin D., Reddy A., Liu M., Murray L., Berger M.F., Monahan J.E., Morais P., Meltzer J., Korejwa A., Jane-Valbuena J., Mapa F.A., Thibault J., Bric-Furlong E., Raman P., Shipway A., Engels I.H., Cheng J., Yu G.-Y.K., Yu J.-J., Aspesi P. Jr., de Silva M., Jagtap K., Jones M.D., Wang L., Hatton C., Palescandolo E., Gupta S., Mahan S., Sougnez C., Onofrio R.C., Liefeld T., MacConaill L.E., Winckler W., Reich M., Li N.-X., Mesirov J.P., Gabriel S.B., Getz G., Ardlie K., Chan V., Myer V.E., Weber B.L., Porter J., Warmuth M., Finan P., Harris J.L., Meyerson M.L., Golub T.R., Morrissey M.P., Sellers W.R., Schlegel R., Garraway L.A.
The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity.
Nature 483:603-607(2012)
PubMed=23401075; DOI=10.1002/path.4176
Guo Y.-N., Chekaluk Y., Zhang J.-M., Du J.-Y., Gray N.S., Wu C.-L., Kwiatkowski D.J.
TSC1 involvement in bladder cancer: diverse effects and therapeutic implications.
J. Pathol. 230:17-27(2013)
PubMed=24367658; DOI=10.1371/journal.pone.0084411; PMCID=PMC3867501
Ross R.L., Burns J.E., Taylor C.F., Mellor P., Anderson D.H., Knowles M.A.
Identification of mutations in distinct regions of p85 alpha in urothelial cancer.
PLoS ONE 8:E84411-E84411(2013)
PubMed=24018021; DOI=10.1016/j.eururo.2013.08.052
Allory Y., Beukers W., Sagrera A., Flandez M., Marques M., Marquez M., van der Keur K.A., Dyrskjot L., Lurkin I., Vermeij M., Carrato A., Lloreta J., Lorente J.A., Carrillo-de-Santa-Pau E., Masius R.G., Kogevinas M., Steyerberg E.W., van Tilborg A.A.G., Abas C., Orntoft T.F., Zuiverloon T.C.M., Malats N., Zwarthoff E.C., Real F.X.
Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome.
Eur. Urol. 65:360-366(2014)
PubMed=24035680; DOI=10.1016/j.eururo.2013.08.057
Hurst C.D., Platt F.M., Knowles M.A.
Comprehensive mutation analysis of the TERT promoter in bladder cancer and detection of mutations in voided urine.
Eur. Urol. 65:367-369(2014)
PubMed=24376083; DOI=10.1002/pmic.201300452
Jeppesen D.K., Nawrocki A., Jensen S.G., Thorsen K., Whitehead B., Howard K.A., Dyrskjot L., Orntoft T.F., Larsen M.R., Ostenfeld M.S.
Quantitative proteomics of fractionated membrane and lumen exosome proteins from isogenic metastatic and nonmetastatic bladder cancer cells reveal differential expression of EMT factors.
Proteomics 14:699-712(2014)
PubMed=24459064; DOI=10.1007/s13277-013-1604-3
Pinto-Leite R., Carreira I.M., Melo J.B., Ferreira S.I., Ribeiro I.P., Ferreira J., Filipe M., Bernardo C., Arantes-Rodrigues R., Oliveira P., Santos L.
Genomic characterization of three urinary bladder cancer cell lines: understanding genomic types of urinary bladder cancer.
Tumor Biol. 35:4599-4617(2014)
PubMed=25997541; DOI=10.1186/s12864-015-1450-3; PMCID=PMC4470036
Earl J., Rico D., Carrillo-de-Santa-Pau E., Rodriguez-Santiago B., Mendez-Pertuz M., Auer H., Gomez G., Grossman H.B., Pisano D.G., Schulz W.A., Perez-Jurado L.A., Carrato A., Theodorescu D., Chanock S.J., Valencia A., Real F.X.
The UBC-40 Urothelial Bladder Cancer cell line index: a genomic resource for functional studies.
BMC Genomics 16:403.1-403.16(2015)
PubMed=26055179; DOI=10.1016/j.tranon.2015.04.002; PMCID=PMC4487788
Vallo S., Michaelis M., Rothweiler F., Bartsch G., Gust K.M., Limbart D.M., Rodel F., Wezel F., Haferkamp A., Cinatl J. Jr.
Drug-resistant urothelial cancer cell lines display diverse sensitivity profiles to potential second-line therapeutics.
Transl. Oncol. 8:210-216(2015)
PubMed=26589293; DOI=10.1186/s13073-015-0240-5; PMCID=PMC4653878
Scholtalbers J., Boegel S., Bukur T., Byl M., Goerges S., Sorn P., Loewer M., Sahin U., Castle J.C.
TCLP: an online cancer cell line catalogue integrating HLA type, predicted neo-epitopes, virus and gene expression.
Genome Med. 7:118.1-118.7(2015)
PubMed=26972028; DOI=10.1016/j.jprot.2016.03.008
Masuishi Y., Kimura Y., Arakawa N., Hirano H.
Identification of glycosylphosphatidylinositol-anchored proteins and omega-sites using TiO2-based affinity purification followed by hydrogen fluoride treatment.
J. Proteomics 139:77-83(2016)
PubMed=27141528; DOI=10.1016/j.dib.2016.04.001; PMCID=PMC4838930
Masuishi Y., Kimura Y., Arakawa N., Hirano H.
Data for identification of GPI-anchored peptides and omega-sites in cancer cell lines.
Data Brief 7:1302-1305(2016)
PubMed=27397505; DOI=10.1016/j.cell.2016.06.017; PMCID=PMC4967469
Iorio F., Knijnenburg T.A., Vis D.J., Bignell G.R., Menden M.P., Schubert M., Aben N., Goncalves E., Barthorpe S., Lightfoot H., Cokelaer T., Greninger P., van Dyk E., Chang H., de Silva H., Heyn H., Deng X.-M., Egan R.K., Liu Q.-S., Miroo T., Mitropoulos X., Richardson L., Wang J.-H., Zhang T.-H., Moran S., Sayols S., Soleimani M., Tamborero D., Lopez-Bigas N., Ross-Macdonald P., Esteller M., Gray N.S., Haber D.A., Stratton M.R., Benes C.H., Wessels L.F.A., Saez-Rodriguez J., McDermott U., Garnett M.J.
A landscape of pharmacogenomic interactions in cancer.
Cell 166:740-754(2016)
PubMed=27270441; DOI=10.1038/onc.2016.172; PMCID=PMC5140783
Nickerson M.L., Witte N., McGee Im K., Turan S., Owens C.R., Misner K., Tsang S.X., Cai Z.-M., Wu S., Dean M., Costello J.C., Theodorescu D.
Molecular analysis of urothelial cancer cell lines for modeling tumor biology and drug response.
Oncogene 36:35-46(2017)
PubMed=28196595; DOI=10.1016/j.ccell.2017.01.005; PMCID=PMC5501076
Li J., Zhao W., Akbani R., Liu W.-B., Ju Z.-L., Ling S.-Y., Vellano C.P., Roebuck P., Yu Q.-H., Eterovic A.K., Byers L.A., Davies M.A., Deng W.-L., Gopal Y.N.V., Chen G., von Euw E.M., Slamon D.J., Conklin D., Heymach J.V., Gazdar A.F., Minna J.D., Myers J.N., Lu Y.-L., Mills G.B., Liang H.
Characterization of human cancer cell lines by reverse-phase protein arrays.
Cancer Cell 31:225-239(2017)
PubMed=29732388; DOI=10.3233/BLC-180167; PMCID=PMC5929350
Zuiverloon T.C.M., de Jong F.C., Costello J.C., Theodorescu D.
Systematic review: characteristics and preclinical uses of bladder cancer cell lines.
Bladder Cancer 4:169-183(2018)
PubMed=30193179; DOI=10.1016/j.jmbbm.2018.08.036
Raczkowska J., Prauzner-Bechcicki S.
Discrimination between HCV29 and T24 by controlled proliferation of cells co-cultured on substrates with different elasticity.
J. Mech. Behav. Biomed. Mater. 88:217-222(2018)
CLPUB00543
Min Q., Cao S.-N., Gan Y.-P., Song T.-S., Liu F.-X., Ning Z.-F.
Bladder cancer chemotherapy resistant cell harbors stem-like characteristics construction and characterization of T24 resistance strain of bladder cancer.
Clin. Oncol. J. 1:1004.09-1004.12(2019)
PubMed=30894373; DOI=10.1158/0008-5472.CAN-18-2747; PMCID=PMC6445675
Dutil J., Chen Z.-H., Monteiro A.N.A., Teer J.K., Eschrich S.A.
An interactive resource to probe genetic diversity and estimated ancestry in cancer cell lines.
Cancer Res. 79:1263-1273(2019)"
风险提示:丁香通仅作为第三方平台,为商家信息发布提供平台空间。用户咨询产品时请注意保护个人信息及财产安全,合理判断,谨慎选购商品,商家和用户对交易行为负责。对于医疗器械类产品,请先查证核实企业经营资质和医疗器械产品注册证情况。
 文献和实验
文献和实验该产品被引用文献
"PubMed=870558; DOI=10.1177/25.4.870558
Benham F.J., Cottell D.C., Franks L.M., Wilson P.D.
Alkaline phosphatase activity in human bladder tumor cell lines.
J. Histochem. Cytochem. 25:266-274(1977)
PubMed=77569; DOI=10.1111/j.1399-0039.1978.tb01259.x
Espmark J.A., Ahlqvist-Roth L., Sarne L., Persson A.
Tissue typing of cells in culture. III. HLA antigens of established human cell lines. Attempts at typing by the mixed hemadsorption technique.
Tissue Antigens 11:279-286(1978)
PubMed=571047
Fogh J.
Cultivation, characterization, and identification of human tumor cells with emphasis on kidney, testis, and bladder tumors.
Natl. Cancer Inst. Monogr. 49:5-9(1978)
PubMed=651066; DOI=10.5980/jpnjurol1928.69.1_40
Kato T., Ishikawa K., Nemoto R.
Morphologic characterization of two established cell lines, T24 and MGH-U1, derived from human bladder carcinoma.
Nihon Hinyokika Gakkai Zasshi 69:40-46(1978)
PubMed=663932; DOI=10.1620/tjem.124.33
Kato T., Ishikawa K., Nemoto R., Senoo A., Amano Y.
Morphological characterization of two established cell lines, T24 and MGH-U1, derived from human urinary bladder carcinoma.
Tohoku J. Exp. Med. 124:339-349(1978)
PubMed=6244232
Williams R.D.
Human urologic cancer cell lines.
Invest. Urol. 17:359-363(1980)
PubMed=7017212; DOI=10.1093/jnci/66.6.1003
Pollack M.S., Heagney S.D., Livingston P.O., Fogh J.
HLA-A, B, C and DR alloantigen expression on forty-six cultured human tumor cell lines.
J. Natl. Cancer Inst. 66:1003-1012(1981)
PubMed=7185004; DOI=10.2302/kjm.31.127
Tachibana M.
Studies on cellular adhesiveness in five different culture cell lines derived from carcinoma of the urinary bladder.
Keio J. Med. 31:127-148(1982)
PubMed=6220172
Dracopoli N.C., Fogh J.
Polymorphic enzyme analysis of cultured human tumor cell lines.
J. Natl. Cancer Inst. 70:469-476(1983)
PubMed=6823318; DOI=10.1038/301429a0
O'Toole C.M., Povey S., Hepburn P.J., Franks L.M.
Identity of some human bladder cancer cell lines.
Nature 301:429-430(1983)
PubMed=6826254; DOI=10.1002/ijc.2910310308
Paulie S., Hansson Y., Lundblad M.-L., Perlmann P.
Lectins as probes for identification of tumor-associated antigens on urothelial and colonic carcinoma cell lines.
Int. J. Cancer 31:297-303(1983)
PubMed=3518877; DOI=10.3109/07357908609038260
Fogh J.
Human tumor lines for cancer research.
Cancer Invest. 4:157-184(1986)
PubMed=3708594
Masters J.R.W., Hepburn P.J., Walker L., Highman W.J., Trejdosiewicz L.K., Povey S., Parkar M., Hill B.T., Riddle P.N., Franks L.M.
Tissue culture model of transitional cell carcinoma: characterization of twenty-two human urothelial cell lines.
Cancer Res. 46:3630-3636(1986)
PubMed=2607719; DOI=10.5980/jpnjurol1989.80.988
Kihara K., Kageyama Y., Sumi S., Higashi Y., Fukui I., Oshima H.
A study of intercellular communication of human transitional cell carcinoma cell lines.
Nihon Hinyokika Gakkai Zasshi 80:988-994(1989)
PubMed=7787250
Cooper M.J., Haluschak J.J., Johnson D., Schwartz S., Morrison L.J., Lippa M., Hatzivassiliou G., Tan J.
p53 mutations in bladder carcinoma cell lines.
Oncol. Res. 6:569-579(1994)
PubMed=8873383; DOI=10.1007/BF00295899
Stadler W.M., Olopade O.I.
The 9p21 region in bladder cancer cell lines: large homozygous deletion inactivate the CDKN2, CDKN2B and MTAP genes.
Urol. Res. 24:239-244(1996)
PubMed=9247707; DOI=10.1080/15216549700202901
Hatakeyama S., Gao Y.-H., Ohara-Nemoto Y., Kataoka H., Satoh M.
Expression of bone morphogenetic proteins of human neoplastic epithelial cells.
Biochem. Mol. Biol. Int. 42:497-505(1997)
PubMed=9290701; DOI=10.1002/(SICI)1098-2744(199708)19:4<243::AID-MC5>3.0.CO;2-D
Jia L.-Q., Osada M., Ishioka C., Gamo M., Ikawa S., Suzuki T., Shimodaira H., Niitani T., Kudo T., Akiyama M., Kimura N., Matsuo M., Mizusawa H., Tanaka N., Koyama H., Namba M., Kanamaru R., Kuroki T.
Screening the p53 status of human cell lines using a yeast functional assay.
Mol. Carcinog. 19:243-253(1997)
PubMed=9850064
Markl I.D.C., Jones P.A.
Presence and location of TP53 mutation determines pattern of CDKN2A/ARF pathway inactivation in bladder cancer.
Cancer Res. 58:5348-5353(1998)
DOI=10.11418/jtca1981.18.4_329
Tanabe H., Takada Y., Minegishi D., Kurematsu M., Masui T., Mizusawa H.
Cell line individualization by STR multiplex system in the cell bank found cross-contamination between ECV304 and EJ-1/T24.
Tissue Cult. Res. Commun. 18:329-338(1999)
PubMed=11416159; DOI=10.1073/pnas.121616198; PMCID=PMC35459
Masters J.R.W., Thomson J.A., Daly-Burns B., Reid Y.A., Dirks W.G., Packer P., Toji L.H., Ohno T., Tanabe H., Arlett C.F., Kelland L.R., Harrison M., Virmani A.K., Ward T.H., Ayres K.L., Debenham P.G.
Short tandem repeat profiling provides an international reference standard for human cell lines.
Proc. Natl. Acad. Sci. U.S.A. 98:8012-8017(2001)
PubMed=11921286; DOI=10.1002/gcc.10050
Williams S.V., Sibley K.D., Davies A.M., Nishiyama H., Hornigold N., Coulter J., Kennedy W.J., Skilleter A., Habuchi T., Knowles M.A.
Molecular genetic analysis of chromosome 9 candidate tumor-suppressor loci in bladder cancer cell lines.
Genes Chromosomes Cancer 34:86-96(2002)
PubMed=12068308; DOI=10.1038/nature00766
Davies H.R., Bignell G.R., Cox C., Stephens P.J., Edkins S., Clegg S., Teague J.W., Woffendin H., Garnett M.J., Bottomley W., Davis N., Dicks E., Ewing R., Floyd Y., Gray K., Hall S., Hawes R., Hughes J., Kosmidou V., Menzies A., Mould C., Parker A., Stevens C., Watt S., Hooper S., Wilson R., Jayatilake H., Gusterson B.A., Cooper C.S., Shipley J.M., Hargrave D., Pritchard-Jones K., Maitland N.J., Chenevix-Trench G., Riggins G.J., Bigner D.D., Palmieri G., Cossu A., Flanagan A.M., Nicholson A., Ho J.W.C., Leung S.Y., Yuen S.T., Weber B.L., Seigler H.F., Darrow T.L., Paterson H.F., Marais R., Marshall C.J., Wooster R., Stratton M.R., Futreal P.A.
Mutations of the BRAF gene in human cancer.
Nature 417:949-954(2002)
PubMed=15846775; DOI=10.1002/gcc.20166
Williams S.V., Adams J., Coulter J., Summersgill B.M., Shipley J.M., Knowles M.A.
Assessment by M-FISH of karyotypic complexity and cytogenetic evolution in bladder cancer in vitro.
Genes Chromosomes Cancer 43:315-328(2005)
PubMed=16885334; DOI=10.1158/0008-5472.CAN-06-1182
Lopez-Knowles E., Hernandez S., Malats N., Kogevinas M., Lloreta J., Carrato A., Tardon A., Serra C., Real F.X.
PIK3CA mutations are an early genetic alteration associated with FGFR3 mutations in superficial papillary bladder tumors.
Cancer Res. 66:7401-7404(2006)
PubMed=19105184; DOI=10.1002/pmic.200800121
Makridakis M., Gagos S., Petrolekas A., Roubelakis M.G., Bitsika V., Stravodimos K., Pavlakis K., Anagnou N.P., Coleman J.A., Vlahou A.
Chromosomal and proteome analysis of a new T24-based cell line model for aggressive bladder cancer.
Proteomics 9:287-298(2009)
PubMed=19375735; DOI=10.1016/j.juro.2009.01.108; PMCID=PMC2680455
Chiong E., Dadbin A., Harris L.D., Sabichi A.L., Grossman H.B.
The use of short tandem repeat profiling to characterize human bladder cancer cell lines.
J. Urol. 181:2737-2748(2009)
PubMed=22460905; DOI=10.1038/nature11003; PMCID=PMC3320027
Barretina J.G., Caponigro G., Stransky N., Venkatesan K., Margolin A.A., Kim S., Wilson C.J., Lehar J., Kryukov G.V., Sonkin D., Reddy A., Liu M., Murray L., Berger M.F., Monahan J.E., Morais P., Meltzer J., Korejwa A., Jane-Valbuena J., Mapa F.A., Thibault J., Bric-Furlong E., Raman P., Shipway A., Engels I.H., Cheng J., Yu G.-Y.K., Yu J.-J., Aspesi P. Jr., de Silva M., Jagtap K., Jones M.D., Wang L., Hatton C., Palescandolo E., Gupta S., Mahan S., Sougnez C., Onofrio R.C., Liefeld T., MacConaill L.E., Winckler W., Reich M., Li N.-X., Mesirov J.P., Gabriel S.B., Getz G., Ardlie K., Chan V., Myer V.E., Weber B.L., Porter J., Warmuth M., Finan P., Harris J.L., Meyerson M.L., Golub T.R., Morrissey M.P., Sellers W.R., Schlegel R., Garraway L.A.
The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity.
Nature 483:603-607(2012)
PubMed=23401075; DOI=10.1002/path.4176
Guo Y.-N., Chekaluk Y., Zhang J.-M., Du J.-Y., Gray N.S., Wu C.-L., Kwiatkowski D.J.
TSC1 involvement in bladder cancer: diverse effects and therapeutic implications.
J. Pathol. 230:17-27(2013)
PubMed=24367658; DOI=10.1371/journal.pone.0084411; PMCID=PMC3867501
Ross R.L., Burns J.E., Taylor C.F., Mellor P., Anderson D.H., Knowles M.A.
Identification of mutations in distinct regions of p85 alpha in urothelial cancer.
PLoS ONE 8:E84411-E84411(2013)
PubMed=24018021; DOI=10.1016/j.eururo.2013.08.052
Allory Y., Beukers W., Sagrera A., Flandez M., Marques M., Marquez M., van der Keur K.A., Dyrskjot L., Lurkin I., Vermeij M., Carrato A., Lloreta J., Lorente J.A., Carrillo-de-Santa-Pau E., Masius R.G., Kogevinas M., Steyerberg E.W., van Tilborg A.A.G., Abas C., Orntoft T.F., Zuiverloon T.C.M., Malats N., Zwarthoff E.C., Real F.X.
Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome.
Eur. Urol. 65:360-366(2014)
PubMed=24035680; DOI=10.1016/j.eururo.2013.08.057
Hurst C.D., Platt F.M., Knowles M.A.
Comprehensive mutation analysis of the TERT promoter in bladder cancer and detection of mutations in voided urine.
Eur. Urol. 65:367-369(2014)
PubMed=24376083; DOI=10.1002/pmic.201300452
Jeppesen D.K., Nawrocki A., Jensen S.G., Thorsen K., Whitehead B., Howard K.A., Dyrskjot L., Orntoft T.F., Larsen M.R., Ostenfeld M.S.
Quantitative proteomics of fractionated membrane and lumen exosome proteins from isogenic metastatic and nonmetastatic bladder cancer cells reveal differential expression of EMT factors.
Proteomics 14:699-712(2014)
PubMed=24459064; DOI=10.1007/s13277-013-1604-3
Pinto-Leite R., Carreira I.M., Melo J.B., Ferreira S.I., Ribeiro I.P., Ferreira J., Filipe M., Bernardo C., Arantes-Rodrigues R., Oliveira P., Santos L.
Genomic characterization of three urinary bladder cancer cell lines: understanding genomic types of urinary bladder cancer.
Tumor Biol. 35:4599-4617(2014)
PubMed=25997541; DOI=10.1186/s12864-015-1450-3; PMCID=PMC4470036
Earl J., Rico D., Carrillo-de-Santa-Pau E., Rodriguez-Santiago B., Mendez-Pertuz M., Auer H., Gomez G., Grossman H.B., Pisano D.G., Schulz W.A., Perez-Jurado L.A., Carrato A., Theodorescu D., Chanock S.J., Valencia A., Real F.X.
The UBC-40 Urothelial Bladder Cancer cell line index: a genomic resource for functional studies.
BMC Genomics 16:403.1-403.16(2015)
PubMed=26055179; DOI=10.1016/j.tranon.2015.04.002; PMCID=PMC4487788
Vallo S., Michaelis M., Rothweiler F., Bartsch G., Gust K.M., Limbart D.M., Rodel F., Wezel F., Haferkamp A., Cinatl J. Jr.
Drug-resistant urothelial cancer cell lines display diverse sensitivity profiles to potential second-line therapeutics.
Transl. Oncol. 8:210-216(2015)
PubMed=26589293; DOI=10.1186/s13073-015-0240-5; PMCID=PMC4653878
Scholtalbers J., Boegel S., Bukur T., Byl M., Goerges S., Sorn P., Loewer M., Sahin U., Castle J.C.
TCLP: an online cancer cell line catalogue integrating HLA type, predicted neo-epitopes, virus and gene expression.
Genome Med. 7:118.1-118.7(2015)
PubMed=26972028; DOI=10.1016/j.jprot.2016.03.008
Masuishi Y., Kimura Y., Arakawa N., Hirano H.
Identification of glycosylphosphatidylinositol-anchored proteins and omega-sites using TiO2-based affinity purification followed by hydrogen fluoride treatment.
J. Proteomics 139:77-83(2016)
PubMed=27141528; DOI=10.1016/j.dib.2016.04.001; PMCID=PMC4838930
Masuishi Y., Kimura Y., Arakawa N., Hirano H.
Data for identification of GPI-anchored peptides and omega-sites in cancer cell lines.
Data Brief 7:1302-1305(2016)
PubMed=27397505; DOI=10.1016/j.cell.2016.06.017; PMCID=PMC4967469
Iorio F., Knijnenburg T.A., Vis D.J., Bignell G.R., Menden M.P., Schubert M., Aben N., Goncalves E., Barthorpe S., Lightfoot H., Cokelaer T., Greninger P., van Dyk E., Chang H., de Silva H., Heyn H., Deng X.-M., Egan R.K., Liu Q.-S., Miroo T., Mitropoulos X., Richardson L., Wang J.-H., Zhang T.-H., Moran S., Sayols S., Soleimani M., Tamborero D., Lopez-Bigas N., Ross-Macdonald P., Esteller M., Gray N.S., Haber D.A., Stratton M.R., Benes C.H., Wessels L.F.A., Saez-Rodriguez J., McDermott U., Garnett M.J.
A landscape of pharmacogenomic interactions in cancer.
Cell 166:740-754(2016)
PubMed=27270441; DOI=10.1038/onc.2016.172; PMCID=PMC5140783
Nickerson M.L., Witte N., McGee Im K., Turan S., Owens C.R., Misner K., Tsang S.X., Cai Z.-M., Wu S., Dean M., Costello J.C., Theodorescu D.
Molecular analysis of urothelial cancer cell lines for modeling tumor biology and drug response.
Oncogene 36:35-46(2017)
PubMed=28196595; DOI=10.1016/j.ccell.2017.01.005; PMCID=PMC5501076
Li J., Zhao W., Akbani R., Liu W.-B., Ju Z.-L., Ling S.-Y., Vellano C.P., Roebuck P., Yu Q.-H., Eterovic A.K., Byers L.A., Davies M.A., Deng W.-L., Gopal Y.N.V., Chen G., von Euw E.M., Slamon D.J., Conklin D., Heymach J.V., Gazdar A.F., Minna J.D., Myers J.N., Lu Y.-L., Mills G.B., Liang H.
Characterization of human cancer cell lines by reverse-phase protein arrays.
Cancer Cell 31:225-239(2017)
PubMed=29732388; DOI=10.3233/BLC-180167; PMCID=PMC5929350
Zuiverloon T.C.M., de Jong F.C., Costello J.C., Theodorescu D.
Systematic review: characteristics and preclinical uses of bladder cancer cell lines.
Bladder Cancer 4:169-183(2018)
PubMed=30193179; DOI=10.1016/j.jmbbm.2018.08.036
Raczkowska J., Prauzner-Bechcicki S.
Discrimination between HCV29 and T24 by controlled proliferation of cells co-cultured on substrates with different elasticity.
J. Mech. Behav. Biomed. Mater. 88:217-222(2018)
CLPUB00543
Min Q., Cao S.-N., Gan Y.-P., Song T.-S., Liu F.-X., Ning Z.-F.
Bladder cancer chemotherapy resistant cell harbors stem-like characteristics construction and characterization of T24 resistance strain of bladder cancer.
Clin. Oncol. J. 1:1004.09-1004.12(2019)
PubMed=30894373; DOI=10.1158/0008-5472.CAN-18-2747; PMCID=PMC6445675
Dutil J., Chen Z.-H., Monteiro A.N.A., Teer J.K., Eschrich S.A.
An interactive resource to probe genetic diversity and estimated ancestry in cancer cell lines.
Cancer Res. 79:1263-1273(2019)"
Benham F.J., Cottell D.C., Franks L.M., Wilson P.D.
Alkaline phosphatase activity in human bladder tumor cell lines.
J. Histochem. Cytochem. 25:266-274(1977)
PubMed=77569; DOI=10.1111/j.1399-0039.1978.tb01259.x
Espmark J.A., Ahlqvist-Roth L., Sarne L., Persson A.
Tissue typing of cells in culture. III. HLA antigens of established human cell lines. Attempts at typing by the mixed hemadsorption technique.
Tissue Antigens 11:279-286(1978)
PubMed=571047
Fogh J.
Cultivation, characterization, and identification of human tumor cells with emphasis on kidney, testis, and bladder tumors.
Natl. Cancer Inst. Monogr. 49:5-9(1978)
PubMed=651066; DOI=10.5980/jpnjurol1928.69.1_40
Kato T., Ishikawa K., Nemoto R.
Morphologic characterization of two established cell lines, T24 and MGH-U1, derived from human bladder carcinoma.
Nihon Hinyokika Gakkai Zasshi 69:40-46(1978)
PubMed=663932; DOI=10.1620/tjem.124.33
Kato T., Ishikawa K., Nemoto R., Senoo A., Amano Y.
Morphological characterization of two established cell lines, T24 and MGH-U1, derived from human urinary bladder carcinoma.
Tohoku J. Exp. Med. 124:339-349(1978)
PubMed=6244232
Williams R.D.
Human urologic cancer cell lines.
Invest. Urol. 17:359-363(1980)
PubMed=7017212; DOI=10.1093/jnci/66.6.1003
Pollack M.S., Heagney S.D., Livingston P.O., Fogh J.
HLA-A, B, C and DR alloantigen expression on forty-six cultured human tumor cell lines.
J. Natl. Cancer Inst. 66:1003-1012(1981)
PubMed=7185004; DOI=10.2302/kjm.31.127
Tachibana M.
Studies on cellular adhesiveness in five different culture cell lines derived from carcinoma of the urinary bladder.
Keio J. Med. 31:127-148(1982)
PubMed=6220172
Dracopoli N.C., Fogh J.
Polymorphic enzyme analysis of cultured human tumor cell lines.
J. Natl. Cancer Inst. 70:469-476(1983)
PubMed=6823318; DOI=10.1038/301429a0
O'Toole C.M., Povey S., Hepburn P.J., Franks L.M.
Identity of some human bladder cancer cell lines.
Nature 301:429-430(1983)
PubMed=6826254; DOI=10.1002/ijc.2910310308
Paulie S., Hansson Y., Lundblad M.-L., Perlmann P.
Lectins as probes for identification of tumor-associated antigens on urothelial and colonic carcinoma cell lines.
Int. J. Cancer 31:297-303(1983)
PubMed=3518877; DOI=10.3109/07357908609038260
Fogh J.
Human tumor lines for cancer research.
Cancer Invest. 4:157-184(1986)
PubMed=3708594
Masters J.R.W., Hepburn P.J., Walker L., Highman W.J., Trejdosiewicz L.K., Povey S., Parkar M., Hill B.T., Riddle P.N., Franks L.M.
Tissue culture model of transitional cell carcinoma: characterization of twenty-two human urothelial cell lines.
Cancer Res. 46:3630-3636(1986)
PubMed=2607719; DOI=10.5980/jpnjurol1989.80.988
Kihara K., Kageyama Y., Sumi S., Higashi Y., Fukui I., Oshima H.
A study of intercellular communication of human transitional cell carcinoma cell lines.
Nihon Hinyokika Gakkai Zasshi 80:988-994(1989)
PubMed=7787250
Cooper M.J., Haluschak J.J., Johnson D., Schwartz S., Morrison L.J., Lippa M., Hatzivassiliou G., Tan J.
p53 mutations in bladder carcinoma cell lines.
Oncol. Res. 6:569-579(1994)
PubMed=8873383; DOI=10.1007/BF00295899
Stadler W.M., Olopade O.I.
The 9p21 region in bladder cancer cell lines: large homozygous deletion inactivate the CDKN2, CDKN2B and MTAP genes.
Urol. Res. 24:239-244(1996)
PubMed=9247707; DOI=10.1080/15216549700202901
Hatakeyama S., Gao Y.-H., Ohara-Nemoto Y., Kataoka H., Satoh M.
Expression of bone morphogenetic proteins of human neoplastic epithelial cells.
Biochem. Mol. Biol. Int. 42:497-505(1997)
PubMed=9290701; DOI=10.1002/(SICI)1098-2744(199708)19:4<243::AID-MC5>3.0.CO;2-D
Jia L.-Q., Osada M., Ishioka C., Gamo M., Ikawa S., Suzuki T., Shimodaira H., Niitani T., Kudo T., Akiyama M., Kimura N., Matsuo M., Mizusawa H., Tanaka N., Koyama H., Namba M., Kanamaru R., Kuroki T.
Screening the p53 status of human cell lines using a yeast functional assay.
Mol. Carcinog. 19:243-253(1997)
PubMed=9850064
Markl I.D.C., Jones P.A.
Presence and location of TP53 mutation determines pattern of CDKN2A/ARF pathway inactivation in bladder cancer.
Cancer Res. 58:5348-5353(1998)
DOI=10.11418/jtca1981.18.4_329
Tanabe H., Takada Y., Minegishi D., Kurematsu M., Masui T., Mizusawa H.
Cell line individualization by STR multiplex system in the cell bank found cross-contamination between ECV304 and EJ-1/T24.
Tissue Cult. Res. Commun. 18:329-338(1999)
PubMed=11416159; DOI=10.1073/pnas.121616198; PMCID=PMC35459
Masters J.R.W., Thomson J.A., Daly-Burns B., Reid Y.A., Dirks W.G., Packer P., Toji L.H., Ohno T., Tanabe H., Arlett C.F., Kelland L.R., Harrison M., Virmani A.K., Ward T.H., Ayres K.L., Debenham P.G.
Short tandem repeat profiling provides an international reference standard for human cell lines.
Proc. Natl. Acad. Sci. U.S.A. 98:8012-8017(2001)
PubMed=11921286; DOI=10.1002/gcc.10050
Williams S.V., Sibley K.D., Davies A.M., Nishiyama H., Hornigold N., Coulter J., Kennedy W.J., Skilleter A., Habuchi T., Knowles M.A.
Molecular genetic analysis of chromosome 9 candidate tumor-suppressor loci in bladder cancer cell lines.
Genes Chromosomes Cancer 34:86-96(2002)
PubMed=12068308; DOI=10.1038/nature00766
Davies H.R., Bignell G.R., Cox C., Stephens P.J., Edkins S., Clegg S., Teague J.W., Woffendin H., Garnett M.J., Bottomley W., Davis N., Dicks E., Ewing R., Floyd Y., Gray K., Hall S., Hawes R., Hughes J., Kosmidou V., Menzies A., Mould C., Parker A., Stevens C., Watt S., Hooper S., Wilson R., Jayatilake H., Gusterson B.A., Cooper C.S., Shipley J.M., Hargrave D., Pritchard-Jones K., Maitland N.J., Chenevix-Trench G., Riggins G.J., Bigner D.D., Palmieri G., Cossu A., Flanagan A.M., Nicholson A., Ho J.W.C., Leung S.Y., Yuen S.T., Weber B.L., Seigler H.F., Darrow T.L., Paterson H.F., Marais R., Marshall C.J., Wooster R., Stratton M.R., Futreal P.A.
Mutations of the BRAF gene in human cancer.
Nature 417:949-954(2002)
PubMed=15846775; DOI=10.1002/gcc.20166
Williams S.V., Adams J., Coulter J., Summersgill B.M., Shipley J.M., Knowles M.A.
Assessment by M-FISH of karyotypic complexity and cytogenetic evolution in bladder cancer in vitro.
Genes Chromosomes Cancer 43:315-328(2005)
PubMed=16885334; DOI=10.1158/0008-5472.CAN-06-1182
Lopez-Knowles E., Hernandez S., Malats N., Kogevinas M., Lloreta J., Carrato A., Tardon A., Serra C., Real F.X.
PIK3CA mutations are an early genetic alteration associated with FGFR3 mutations in superficial papillary bladder tumors.
Cancer Res. 66:7401-7404(2006)
PubMed=19105184; DOI=10.1002/pmic.200800121
Makridakis M., Gagos S., Petrolekas A., Roubelakis M.G., Bitsika V., Stravodimos K., Pavlakis K., Anagnou N.P., Coleman J.A., Vlahou A.
Chromosomal and proteome analysis of a new T24-based cell line model for aggressive bladder cancer.
Proteomics 9:287-298(2009)
PubMed=19375735; DOI=10.1016/j.juro.2009.01.108; PMCID=PMC2680455
Chiong E., Dadbin A., Harris L.D., Sabichi A.L., Grossman H.B.
The use of short tandem repeat profiling to characterize human bladder cancer cell lines.
J. Urol. 181:2737-2748(2009)
PubMed=22460905; DOI=10.1038/nature11003; PMCID=PMC3320027
Barretina J.G., Caponigro G., Stransky N., Venkatesan K., Margolin A.A., Kim S., Wilson C.J., Lehar J., Kryukov G.V., Sonkin D., Reddy A., Liu M., Murray L., Berger M.F., Monahan J.E., Morais P., Meltzer J., Korejwa A., Jane-Valbuena J., Mapa F.A., Thibault J., Bric-Furlong E., Raman P., Shipway A., Engels I.H., Cheng J., Yu G.-Y.K., Yu J.-J., Aspesi P. Jr., de Silva M., Jagtap K., Jones M.D., Wang L., Hatton C., Palescandolo E., Gupta S., Mahan S., Sougnez C., Onofrio R.C., Liefeld T., MacConaill L.E., Winckler W., Reich M., Li N.-X., Mesirov J.P., Gabriel S.B., Getz G., Ardlie K., Chan V., Myer V.E., Weber B.L., Porter J., Warmuth M., Finan P., Harris J.L., Meyerson M.L., Golub T.R., Morrissey M.P., Sellers W.R., Schlegel R., Garraway L.A.
The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity.
Nature 483:603-607(2012)
PubMed=23401075; DOI=10.1002/path.4176
Guo Y.-N., Chekaluk Y., Zhang J.-M., Du J.-Y., Gray N.S., Wu C.-L., Kwiatkowski D.J.
TSC1 involvement in bladder cancer: diverse effects and therapeutic implications.
J. Pathol. 230:17-27(2013)
PubMed=24367658; DOI=10.1371/journal.pone.0084411; PMCID=PMC3867501
Ross R.L., Burns J.E., Taylor C.F., Mellor P., Anderson D.H., Knowles M.A.
Identification of mutations in distinct regions of p85 alpha in urothelial cancer.
PLoS ONE 8:E84411-E84411(2013)
PubMed=24018021; DOI=10.1016/j.eururo.2013.08.052
Allory Y., Beukers W., Sagrera A., Flandez M., Marques M., Marquez M., van der Keur K.A., Dyrskjot L., Lurkin I., Vermeij M., Carrato A., Lloreta J., Lorente J.A., Carrillo-de-Santa-Pau E., Masius R.G., Kogevinas M., Steyerberg E.W., van Tilborg A.A.G., Abas C., Orntoft T.F., Zuiverloon T.C.M., Malats N., Zwarthoff E.C., Real F.X.
Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome.
Eur. Urol. 65:360-366(2014)
PubMed=24035680; DOI=10.1016/j.eururo.2013.08.057
Hurst C.D., Platt F.M., Knowles M.A.
Comprehensive mutation analysis of the TERT promoter in bladder cancer and detection of mutations in voided urine.
Eur. Urol. 65:367-369(2014)
PubMed=24376083; DOI=10.1002/pmic.201300452
Jeppesen D.K., Nawrocki A., Jensen S.G., Thorsen K., Whitehead B., Howard K.A., Dyrskjot L., Orntoft T.F., Larsen M.R., Ostenfeld M.S.
Quantitative proteomics of fractionated membrane and lumen exosome proteins from isogenic metastatic and nonmetastatic bladder cancer cells reveal differential expression of EMT factors.
Proteomics 14:699-712(2014)
PubMed=24459064; DOI=10.1007/s13277-013-1604-3
Pinto-Leite R., Carreira I.M., Melo J.B., Ferreira S.I., Ribeiro I.P., Ferreira J., Filipe M., Bernardo C., Arantes-Rodrigues R., Oliveira P., Santos L.
Genomic characterization of three urinary bladder cancer cell lines: understanding genomic types of urinary bladder cancer.
Tumor Biol. 35:4599-4617(2014)
PubMed=25997541; DOI=10.1186/s12864-015-1450-3; PMCID=PMC4470036
Earl J., Rico D., Carrillo-de-Santa-Pau E., Rodriguez-Santiago B., Mendez-Pertuz M., Auer H., Gomez G., Grossman H.B., Pisano D.G., Schulz W.A., Perez-Jurado L.A., Carrato A., Theodorescu D., Chanock S.J., Valencia A., Real F.X.
The UBC-40 Urothelial Bladder Cancer cell line index: a genomic resource for functional studies.
BMC Genomics 16:403.1-403.16(2015)
PubMed=26055179; DOI=10.1016/j.tranon.2015.04.002; PMCID=PMC4487788
Vallo S., Michaelis M., Rothweiler F., Bartsch G., Gust K.M., Limbart D.M., Rodel F., Wezel F., Haferkamp A., Cinatl J. Jr.
Drug-resistant urothelial cancer cell lines display diverse sensitivity profiles to potential second-line therapeutics.
Transl. Oncol. 8:210-216(2015)
PubMed=26589293; DOI=10.1186/s13073-015-0240-5; PMCID=PMC4653878
Scholtalbers J., Boegel S., Bukur T., Byl M., Goerges S., Sorn P., Loewer M., Sahin U., Castle J.C.
TCLP: an online cancer cell line catalogue integrating HLA type, predicted neo-epitopes, virus and gene expression.
Genome Med. 7:118.1-118.7(2015)
PubMed=26972028; DOI=10.1016/j.jprot.2016.03.008
Masuishi Y., Kimura Y., Arakawa N., Hirano H.
Identification of glycosylphosphatidylinositol-anchored proteins and omega-sites using TiO2-based affinity purification followed by hydrogen fluoride treatment.
J. Proteomics 139:77-83(2016)
PubMed=27141528; DOI=10.1016/j.dib.2016.04.001; PMCID=PMC4838930
Masuishi Y., Kimura Y., Arakawa N., Hirano H.
Data for identification of GPI-anchored peptides and omega-sites in cancer cell lines.
Data Brief 7:1302-1305(2016)
PubMed=27397505; DOI=10.1016/j.cell.2016.06.017; PMCID=PMC4967469
Iorio F., Knijnenburg T.A., Vis D.J., Bignell G.R., Menden M.P., Schubert M., Aben N., Goncalves E., Barthorpe S., Lightfoot H., Cokelaer T., Greninger P., van Dyk E., Chang H., de Silva H., Heyn H., Deng X.-M., Egan R.K., Liu Q.-S., Miroo T., Mitropoulos X., Richardson L., Wang J.-H., Zhang T.-H., Moran S., Sayols S., Soleimani M., Tamborero D., Lopez-Bigas N., Ross-Macdonald P., Esteller M., Gray N.S., Haber D.A., Stratton M.R., Benes C.H., Wessels L.F.A., Saez-Rodriguez J., McDermott U., Garnett M.J.
A landscape of pharmacogenomic interactions in cancer.
Cell 166:740-754(2016)
PubMed=27270441; DOI=10.1038/onc.2016.172; PMCID=PMC5140783
Nickerson M.L., Witte N., McGee Im K., Turan S., Owens C.R., Misner K., Tsang S.X., Cai Z.-M., Wu S., Dean M., Costello J.C., Theodorescu D.
Molecular analysis of urothelial cancer cell lines for modeling tumor biology and drug response.
Oncogene 36:35-46(2017)
PubMed=28196595; DOI=10.1016/j.ccell.2017.01.005; PMCID=PMC5501076
Li J., Zhao W., Akbani R., Liu W.-B., Ju Z.-L., Ling S.-Y., Vellano C.P., Roebuck P., Yu Q.-H., Eterovic A.K., Byers L.A., Davies M.A., Deng W.-L., Gopal Y.N.V., Chen G., von Euw E.M., Slamon D.J., Conklin D., Heymach J.V., Gazdar A.F., Minna J.D., Myers J.N., Lu Y.-L., Mills G.B., Liang H.
Characterization of human cancer cell lines by reverse-phase protein arrays.
Cancer Cell 31:225-239(2017)
PubMed=29732388; DOI=10.3233/BLC-180167; PMCID=PMC5929350
Zuiverloon T.C.M., de Jong F.C., Costello J.C., Theodorescu D.
Systematic review: characteristics and preclinical uses of bladder cancer cell lines.
Bladder Cancer 4:169-183(2018)
PubMed=30193179; DOI=10.1016/j.jmbbm.2018.08.036
Raczkowska J., Prauzner-Bechcicki S.
Discrimination between HCV29 and T24 by controlled proliferation of cells co-cultured on substrates with different elasticity.
J. Mech. Behav. Biomed. Mater. 88:217-222(2018)
CLPUB00543
Min Q., Cao S.-N., Gan Y.-P., Song T.-S., Liu F.-X., Ning Z.-F.
Bladder cancer chemotherapy resistant cell harbors stem-like characteristics construction and characterization of T24 resistance strain of bladder cancer.
Clin. Oncol. J. 1:1004.09-1004.12(2019)
PubMed=30894373; DOI=10.1158/0008-5472.CAN-18-2747; PMCID=PMC6445675
Dutil J., Chen Z.-H., Monteiro A.N.A., Teer J.K., Eschrich S.A.
An interactive resource to probe genetic diversity and estimated ancestry in cancer cell lines.
Cancer Res. 79:1263-1273(2019)"
文献支持
T24[T-24]人膀胱移行细胞癌传代细胞长期复苏|送STR图谱
¥850 - 2150


![T24[T-24]人膀胱移行细胞癌传代细胞长期复苏|送STR图谱](https://img1.dxycdn.com/p/s14/2025/0213/627/0276235109246789881.jpg)


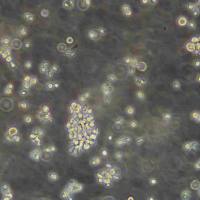

![LLC-WRC 256[Walker 256]大鼠腹水癌传代细胞|送STR图谱](https://img1.dxycdn.com/p/s14/2025/0209/192/2179315283446358881.jpg!wh200)


