相关产品推荐更多 >
万千商家帮你免费找货
0 人在求购买到急需产品
- 详细信息
- 询价记录
- 文献和实验
- 技术资料
产品用途:将鼠固定在操作台上,结合大小鼠插管的内窥可视喉镜,通过该雾化针可以将精确定量的液体、粉末供试品雾化给到大小鼠的肺部。
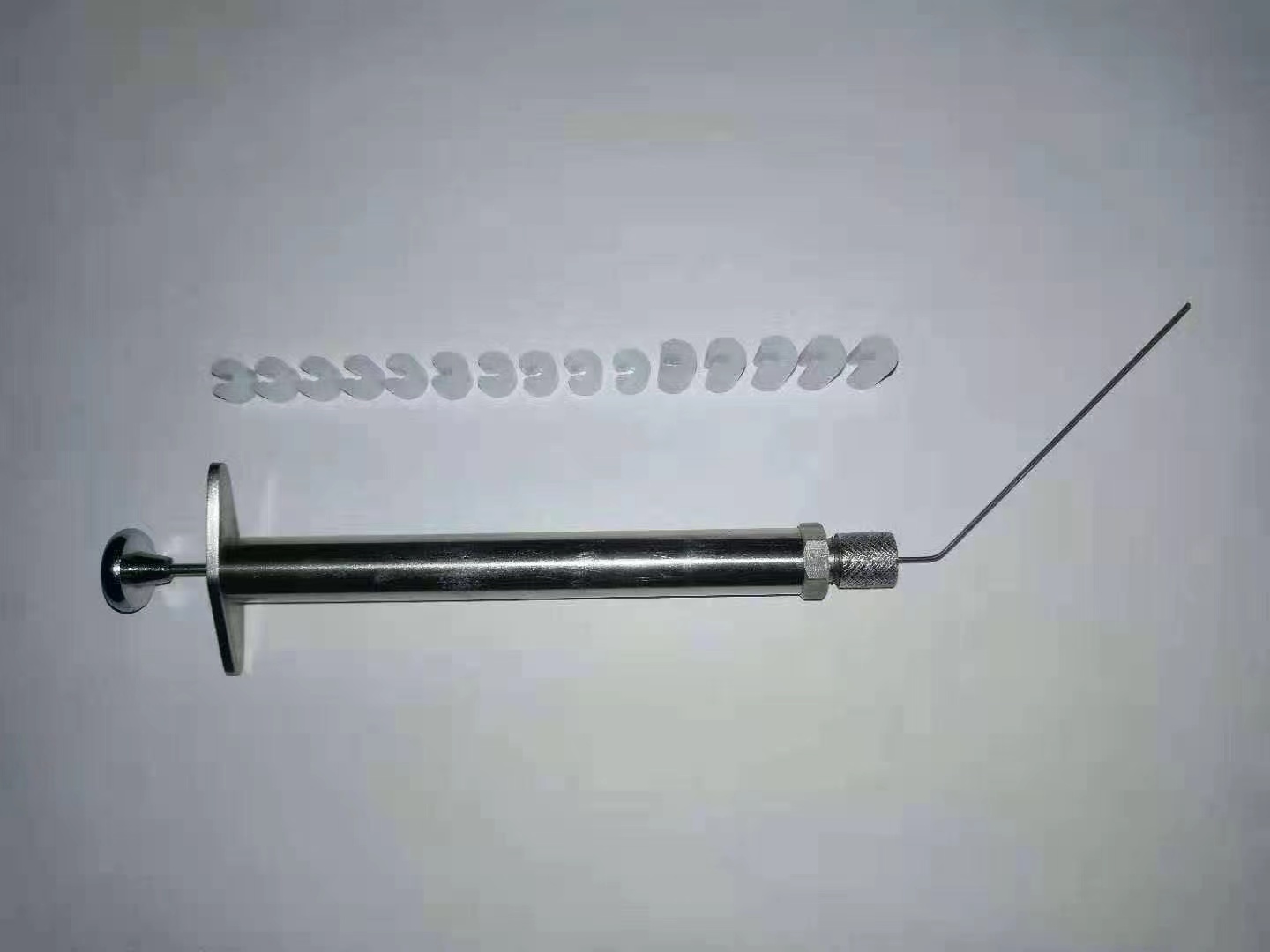
性能特点:
精确定量
较气管内滴入在各肺叶中分布更均匀
直达肺部、易于操作
更安全的提供高浓度
可输送液体、干粉样品
应用范围:
广泛应用于呼吸系统疾病、毒理学、药理学、吸入免疫、生物安全、大气污染物、化学物质毒性鉴定、药物开发与安全性评价、环境与健康等领域

性能特点:
精确定量
较气管内滴入在各肺叶中分布更均匀
直达肺部、易于操作
更安全的提供高浓度
可输送液体、干粉样品
应用范围:
广泛应用于呼吸系统疾病、毒理学、药理学、吸入免疫、生物安全、大气污染物、化学物质毒性鉴定、药物开发与安全性评价、环境与健康等领域
风险提示:丁香通仅作为第三方平台,为商家信息发布提供平台空间。用户咨询产品时请注意保护个人信息及财产安全,合理判断,谨慎选购商品,商家和用户对交易行为负责。对于医疗器械类产品,请先查证核实企业经营资质和医疗器械产品注册证情况。
- 作者
- 内容
- 询问日期
 文献和实验
文献和实验该产品被引用文献
Inhalation of MSC-EVs is a noninvasive strategy for ameliorating acute
lung injury
Ruijing Zhao a,1
, Lina Wang a,1
, Tian Wang a,b
, Panpan Xian a
, Hongkang Wang a
,
Qianfa Long a,c,*
a Mini-invasive Neurosurgery and Translational Medical Center, Xi’an Central Hospital, Xi’an Jiaotong University. No. 161, West 5th Road, Xincheng District, Xi’an
710003, China b Shaanxi Lon-EV Biotechnology Limited Company, No.9 Jiazi, Renyi village, Beilin District, Xi’an 710054, China c College of Medicine, Yan’an University, Yongxiang Road, Baota District, Yan’an 716000, China
ARTICLE INFO
Keywords:
Small extracellular vesicles
Inhalation
Acute lung injury
Immunomodulation
Redox system
ABSTRACT
Mesenchymal stem cell-derived small extracellular vesicles (MSC-EVs) are promising nanotherapeutic agent for
pneumonia (bacterial origin, COVID-19), but the optimal administration route and potential mechanisms of
action remain poorly understood. This study compared the administration of MSC-EVs via inhalation and tail
vein injection for the treatment of acute lung injury (ALI) and determined the host-derived mechanisms that may
contribute to the therapeutic effects of MSC-EVs in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells
(macrophage cell line) and animal models. Luminex liquid chip and hematoxylin and eosin (HE) staining
revealed that, compared with the vehicle control, inhaled MSC-EVs outperformed those injected via the tail vein,
by reducing the expression of pro-inflammatory cytokines, increasing the expression of anti-inflammatory
cytokine, and decreasing pathological scores in ALI. MSC-EV administration promoted the polarization of
macrophages towards a M2 phenotype in vitro and in vivo (via inhalation). RNA sequencing revealed that immune
and redox mediators, including TLR4, Arg1, and HO-1, were associated with the activity MSC-EVs against ALI
mice. Western blotting and immunofluorescence revealed that correlative inflammatory and oxidative mediators
were involved in the therapeutic effects of MSC-EVs in LPS-stimulated cells and mice. Moreover, variable
expression of Nrf2 was observed following treatment with MSC-EVs in cell and animal models, and knockdown of
Nrf2 attenuated the anti-inflammatory and antioxidant activities of MSC-EVs in LPS-stimulated macrophages.
Together, these data suggest that inhalation of MSC-EVs as a noninvasive strategy for attenuation of ALI, and the
adaptive regulation of Nrf2 may contribute to their anti-inflammatory and anti-oxidant activity in mice.
1. Introduction
Acute lung injury (ALI) is characterized by impaired pulmonary gas
exchange, bilateral infiltrates, and noncardiogenic edema, and can be
induced by direct injury and systemic stimuli, such as mechanical
trauma, bacteria, and viruses (e.g., SARS-CoV-2), resulting in human and
economic burden [1,2]. If pulmonary disease is not effectively managed
during the early stage, acute respiratory distress syndrome (ARDS) can
develop, and is associated with high mortality [3]. ALI is associated with
severe acute inflammation, as well as the complications of infections,
such as increased permeability of blood vessels and the death of
pulmonary epithelial and endothelial cells [2]. Stem cell therapy has
demonstrated great potential in the treatment of lung injury, including
that induced by COVID-19, owing to its immunomodulatory and tissue
repair properties [4,5]. However, the cellular candidates, optimal
management, and therapeutic mechanisms are not well understood.
Increasing evidence, including data from our previous studies, suggests that small extracellular vesicles (EVs) exhibit more potential than
their parental cells (e.g. MSCs) as therapeutics against inflammatory
diseases, owing to characteristics such as blood-air barrier permeability,
freeze / thaw resistance, and targeting to injured cells [6,7]. Recent
studies have shown that EV-based therapies hold potential for the
* Corresponding author at: Mini-invasive Neurosurgery and Translational Medical Center, Xi’an Central Hospital, Xi’an Jiaotong University, No. 161, West 5th
Road, Xincheng District, Xi’an 710003, China.
E-mail address: lonva@live.cn (Q. Long). 1 These authors contributed this work equally.
Contents lists available at ScienceDirect
Journal of Controlled Release
journal homepage: www.elsevier.com/locate/jconrel
https://doi.org/10.1016/j.jconrel.2022.03.025
Received 18 November 2021; Received in revised form 21 February 2022; Accepted 14 March 2022 Journal of Controlled Release 345 (2022) 214–230
215
treatment of lung injury, such as that induced by COVID-19, as they can
target multiple pathways and enhance tissue regeneration [8]. In addition, MSC-EVs can attenuate ALI via mitochondrial or miRNA transfer
and modulate macrophage polarization because they contain multiple
functional cargoes, including proteins, lipids, RNA, and metabolites
[6,9,10]. Besides to determine the therapeutic agents carried by EVs, it
also is important to elucidate the therapeutic mechanisms, including
immunomodulation, antioxidation, and tissue regeneration in target
cells or injured tissues during ALI.
Inflammatory stimuli can evoke the excessive production of reactive
oxygen species (ROS) in pulmonary tissue, followed by the development
of ALI. Oxidative stress is an early contributor to ALI, and can cause
macrophage activation, cellular infiltration, and enhanced pulmonary
cytokine production [11,12]. Thus, crosstalk between oxidation and
inflammation is important for regulating the initiation and progression
of ALI. Specifically, the nuclear factor kappa beta (NF-κB) pathway is
activated by a variety of stimuli in ALI, and activation of NF-κB downstream of Toll-like receptor 4 (TLR4) and transcription factors such as
signal transducer and activator of transcription 3 (STAT3) mediates
macrophage plasticity and inflammation [13]. Also, the antiinflammatory or antioxidative activity in ALI depends on the regulation of nuclear factor erythroid 2-related factor 2 (Nrf2, a key mediator
in oxidative stress) [12,14]. Together, these mediators may orchestrate
inflammation and oxidation during lung injury. Previously, we showed
that MSC-EVs exert significant biological activities in models of
inflammation and oxidative stress [15,16], which suggests that EV
therapy may have potential to regulate the crosstalk between oxidation
and inflammation in ALI.
In the present study, inhalation and tail vein injection were used as
administration methods to examine the potential activity of MSC-EVs in
mice with ALI. This was followed by RNA sequencing (RNA-Seq), molecular pattern tests, and Nrf2 knockdown to elucidate the therapeutic
mechanism of MSC-EVs in lipopolysaccharide (LPS)-stimulated cells or
mice. The results showed that administration of MSC-EVs via inhalation
has potential against acute lung inflammation and oxidation, highlighting the clinical value of MSC-EV inhalation in ALI, even in that
induced by COVID-19.
2. Materials and methods (Fig. S1)
2.1. Cell preparation
For preparation of allogeneic MSCs, informed consent was obtained
before cell collection, and 3 donors (age 27–29) were selected from fullterm puerpera in good health. All procedures were approved by the
Ethical Committee of the Xi’an Central Hospital, Xi’an Jiaotong University, as well as in accordance with the Guidelines of the National
Institutes of Health. MSCs were obtained from Wharton’s jelly in the
umbilical cord and characterized by flow cytometry, the gating strategy
was employed by using Fluorescence Minus One control, as in our previous reports [15,17]. Primary antibodies including rabbit polyclonal
anti-CD105, CD90, CD73, CD45, CD34 and CD11b (1:100 dilution)
(Bioss, Wuhan, CHN), and secondary antibody Alexa Fluor 488 goat
anti-rabbit IgG (1:500) (Invitrogen, A-21206, CA, USA) were used to
detect the surface antigens of fifth-passage MSCs. At least three cell
culture samples were examined on an FACS Calibur instrument (Becton
Dickinson) and the data were analyzed using Cell Quest software (Becton Dickinson). Multi-potency of MSCs was detected by StemPro®
Osteogenesis (Gibco, A1007201, MD, USA), Chondrogenesis (Gibco,
A1007101, MD, USA) and Adipogenesis (Gibco, A1007001, MD, USA)
differentiation Kits (37 ◦C, 5% CO2) according to the instructions. RAW
264.7 cells, a murine macrophage cell line, were purchased from the Cell
Bank of Type Culture Collection of Chinese Academy of Sciences
(Shanghai, China) and cultured in Dulbecco’s modified Eagle medium
(DMEM) / F12 + 10% fetal bovine serum (FBS) at 37 ◦C, 5% CO2.
2.2. Isolation, characterization and labelling of MSC-EVs
EVs were isolated from the supernatants of fifth-passage MSCs, as
previously described [15]. Briefly, the ratio of live and dead MSCs was
detected by using an automatic cell counter (Bodboge, Shenzhen, CHN),
the batch of which contained more than 99% live cells. The MSCs were
cultured in αMEM containing 10% EV-depleted FBS for 24 h, and then
the supernatants were harvested and processed via a series of centrifugation steps (300 ×g for 10 min, 2000 ×g for 10 min, and 10,000 ×g for
30 min; ST16R, Thermo Fisher, USA). Subsequently, the EVs were
collected via ultracentrifugation at 100,000 ×g for 70 min (XPN-100,
Beckman Coulter, USA). The nanoparticles were then characterized by
western blotting based on the positive markers TSG101 and CD9, as well
as negative marker calnexin, and examined via transmission electron
microscopy (TEM) and nanoparticle tracking analysis (NTA) to evaluate
morphology and size distribution, respectively. In addition, C5
Maleimide-Alexa 594 (CM-A954) (Invitrogen, A10256, California, USA)
was used to label MSC-EVs as our previous reports [17].
2.3. Macrophage activation and intervention
RAW 264.7 cells were pretreated with 10 μg / mL MSC-EVs
(Fig. S2A) for 12 h to ensure uptake, and then activated with 100 ng /
mL lipopolysaccharide (LPS; L2880, Sigma-Aldrich, CA, USA) for 12 h,
as previously reported [18]. ML385 (15 μM; HY-100523, Medchem
Express, New Jersey, USA), a pharmacological inhibitor of Nrf2, was
used to downregulate Nrf2 expression in RAW 264.7 cells.
2.4. Animal procedures
97 adult male (8–10-weeks old) C57BL/6 mice were purchased from
the Experimental Animal Center of Xi’an Jiaotong University, they were
housed in groups and were allowed a period to acclimatize to the laboratory environment before the start of the study. All animal procedures
were performed in accordance with the ARRIVE guidelines and
approved by the Ethics Review Board of Xi’an Central Hospital, Xi’an
Jiaotong University. Animals were housed under a controlled environment with a 12 / 12 h light / dark cycle with food and water provided.
Mice were intraperitoneally anesthetized using 4.0% chloralhydrate
(10 mL / kg) and administered LPS (10 mg / kg, diluted with saline)
intratracheally. A sham operation was performed in a similar manner
using saline solution (Sham group, n = 5). After LPS induction for 3 h,
50 μg MSC-EVs (diluted in 50 μL saline, Fig. S2B and C) and 50 μL saline
(vehicle) were administered via inhalation using an atomizer (YSKD BioTec, Beijing, China) as the ALI-inh + EVs (n = 20) and ALI-inh + Veh (n
= 15) groups, or administered by tail vein injection as the ALI-iv + EVs
(n = 15) and ALI-iv + Veh (n = 15) groups, respectively. The mice were
sacrificed at random via isoflurane at 24 h, 4 days (d), and 14 d after EV
administration, and lung tissues and blood samples were collected for
further analyses (Fig. S1). Also, to track the MSC-EVs in vitro, ALI mice
received MSC-EVs via inhalation (negative control, n = 3), or CM-A594
labeled MSC-EVs via inhalation (n = 3) and tail vein injection (n = 3) for
24 h, the lung tissues were then processed as above procedures. Additionally, 8 mice were excluded due to failed injection via tail vein, and
10 mice were died in the present experiments.
2.5. Luminex liquid chip
Following treatment with MSC-EVs for 24 h, whole blood was
collected from mouse orbits and centrifuged (10,000 rpm) for 10 min.
Supernatants from each group were assayed using a Luminex liquid chip
(Luminex 200, USA) for interleukin (IL)-1β, macrophage chemoattractant protein-1 (MCP-1), IL-1α, tumor necrosis factor (TNF)α, IL-12,
and IL-10 (MHSTCMAG-70 K, Mouse High Sensitivity T Cell Magnetic
lung injury
Ruijing Zhao a,1
, Lina Wang a,1
, Tian Wang a,b
, Panpan Xian a
, Hongkang Wang a
,
Qianfa Long a,c,*
a Mini-invasive Neurosurgery and Translational Medical Center, Xi’an Central Hospital, Xi’an Jiaotong University. No. 161, West 5th Road, Xincheng District, Xi’an
710003, China b Shaanxi Lon-EV Biotechnology Limited Company, No.9 Jiazi, Renyi village, Beilin District, Xi’an 710054, China c College of Medicine, Yan’an University, Yongxiang Road, Baota District, Yan’an 716000, China
ARTICLE INFO
Keywords:
Small extracellular vesicles
Inhalation
Acute lung injury
Immunomodulation
Redox system
ABSTRACT
Mesenchymal stem cell-derived small extracellular vesicles (MSC-EVs) are promising nanotherapeutic agent for
pneumonia (bacterial origin, COVID-19), but the optimal administration route and potential mechanisms of
action remain poorly understood. This study compared the administration of MSC-EVs via inhalation and tail
vein injection for the treatment of acute lung injury (ALI) and determined the host-derived mechanisms that may
contribute to the therapeutic effects of MSC-EVs in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells
(macrophage cell line) and animal models. Luminex liquid chip and hematoxylin and eosin (HE) staining
revealed that, compared with the vehicle control, inhaled MSC-EVs outperformed those injected via the tail vein,
by reducing the expression of pro-inflammatory cytokines, increasing the expression of anti-inflammatory
cytokine, and decreasing pathological scores in ALI. MSC-EV administration promoted the polarization of
macrophages towards a M2 phenotype in vitro and in vivo (via inhalation). RNA sequencing revealed that immune
and redox mediators, including TLR4, Arg1, and HO-1, were associated with the activity MSC-EVs against ALI
mice. Western blotting and immunofluorescence revealed that correlative inflammatory and oxidative mediators
were involved in the therapeutic effects of MSC-EVs in LPS-stimulated cells and mice. Moreover, variable
expression of Nrf2 was observed following treatment with MSC-EVs in cell and animal models, and knockdown of
Nrf2 attenuated the anti-inflammatory and antioxidant activities of MSC-EVs in LPS-stimulated macrophages.
Together, these data suggest that inhalation of MSC-EVs as a noninvasive strategy for attenuation of ALI, and the
adaptive regulation of Nrf2 may contribute to their anti-inflammatory and anti-oxidant activity in mice.
1. Introduction
Acute lung injury (ALI) is characterized by impaired pulmonary gas
exchange, bilateral infiltrates, and noncardiogenic edema, and can be
induced by direct injury and systemic stimuli, such as mechanical
trauma, bacteria, and viruses (e.g., SARS-CoV-2), resulting in human and
economic burden [1,2]. If pulmonary disease is not effectively managed
during the early stage, acute respiratory distress syndrome (ARDS) can
develop, and is associated with high mortality [3]. ALI is associated with
severe acute inflammation, as well as the complications of infections,
such as increased permeability of blood vessels and the death of
pulmonary epithelial and endothelial cells [2]. Stem cell therapy has
demonstrated great potential in the treatment of lung injury, including
that induced by COVID-19, owing to its immunomodulatory and tissue
repair properties [4,5]. However, the cellular candidates, optimal
management, and therapeutic mechanisms are not well understood.
Increasing evidence, including data from our previous studies, suggests that small extracellular vesicles (EVs) exhibit more potential than
their parental cells (e.g. MSCs) as therapeutics against inflammatory
diseases, owing to characteristics such as blood-air barrier permeability,
freeze / thaw resistance, and targeting to injured cells [6,7]. Recent
studies have shown that EV-based therapies hold potential for the
* Corresponding author at: Mini-invasive Neurosurgery and Translational Medical Center, Xi’an Central Hospital, Xi’an Jiaotong University, No. 161, West 5th
Road, Xincheng District, Xi’an 710003, China.
E-mail address: lonva@live.cn (Q. Long). 1 These authors contributed this work equally.
Contents lists available at ScienceDirect
Journal of Controlled Release
journal homepage: www.elsevier.com/locate/jconrel
https://doi.org/10.1016/j.jconrel.2022.03.025
Received 18 November 2021; Received in revised form 21 February 2022; Accepted 14 March 2022 Journal of Controlled Release 345 (2022) 214–230
215
treatment of lung injury, such as that induced by COVID-19, as they can
target multiple pathways and enhance tissue regeneration [8]. In addition, MSC-EVs can attenuate ALI via mitochondrial or miRNA transfer
and modulate macrophage polarization because they contain multiple
functional cargoes, including proteins, lipids, RNA, and metabolites
[6,9,10]. Besides to determine the therapeutic agents carried by EVs, it
also is important to elucidate the therapeutic mechanisms, including
immunomodulation, antioxidation, and tissue regeneration in target
cells or injured tissues during ALI.
Inflammatory stimuli can evoke the excessive production of reactive
oxygen species (ROS) in pulmonary tissue, followed by the development
of ALI. Oxidative stress is an early contributor to ALI, and can cause
macrophage activation, cellular infiltration, and enhanced pulmonary
cytokine production [11,12]. Thus, crosstalk between oxidation and
inflammation is important for regulating the initiation and progression
of ALI. Specifically, the nuclear factor kappa beta (NF-κB) pathway is
activated by a variety of stimuli in ALI, and activation of NF-κB downstream of Toll-like receptor 4 (TLR4) and transcription factors such as
signal transducer and activator of transcription 3 (STAT3) mediates
macrophage plasticity and inflammation [13]. Also, the antiinflammatory or antioxidative activity in ALI depends on the regulation of nuclear factor erythroid 2-related factor 2 (Nrf2, a key mediator
in oxidative stress) [12,14]. Together, these mediators may orchestrate
inflammation and oxidation during lung injury. Previously, we showed
that MSC-EVs exert significant biological activities in models of
inflammation and oxidative stress [15,16], which suggests that EV
therapy may have potential to regulate the crosstalk between oxidation
and inflammation in ALI.
In the present study, inhalation and tail vein injection were used as
administration methods to examine the potential activity of MSC-EVs in
mice with ALI. This was followed by RNA sequencing (RNA-Seq), molecular pattern tests, and Nrf2 knockdown to elucidate the therapeutic
mechanism of MSC-EVs in lipopolysaccharide (LPS)-stimulated cells or
mice. The results showed that administration of MSC-EVs via inhalation
has potential against acute lung inflammation and oxidation, highlighting the clinical value of MSC-EV inhalation in ALI, even in that
induced by COVID-19.
2. Materials and methods (Fig. S1)
2.1. Cell preparation
For preparation of allogeneic MSCs, informed consent was obtained
before cell collection, and 3 donors (age 27–29) were selected from fullterm puerpera in good health. All procedures were approved by the
Ethical Committee of the Xi’an Central Hospital, Xi’an Jiaotong University, as well as in accordance with the Guidelines of the National
Institutes of Health. MSCs were obtained from Wharton’s jelly in the
umbilical cord and characterized by flow cytometry, the gating strategy
was employed by using Fluorescence Minus One control, as in our previous reports [15,17]. Primary antibodies including rabbit polyclonal
anti-CD105, CD90, CD73, CD45, CD34 and CD11b (1:100 dilution)
(Bioss, Wuhan, CHN), and secondary antibody Alexa Fluor 488 goat
anti-rabbit IgG (1:500) (Invitrogen, A-21206, CA, USA) were used to
detect the surface antigens of fifth-passage MSCs. At least three cell
culture samples were examined on an FACS Calibur instrument (Becton
Dickinson) and the data were analyzed using Cell Quest software (Becton Dickinson). Multi-potency of MSCs was detected by StemPro®
Osteogenesis (Gibco, A1007201, MD, USA), Chondrogenesis (Gibco,
A1007101, MD, USA) and Adipogenesis (Gibco, A1007001, MD, USA)
differentiation Kits (37 ◦C, 5% CO2) according to the instructions. RAW
264.7 cells, a murine macrophage cell line, were purchased from the Cell
Bank of Type Culture Collection of Chinese Academy of Sciences
(Shanghai, China) and cultured in Dulbecco’s modified Eagle medium
(DMEM) / F12 + 10% fetal bovine serum (FBS) at 37 ◦C, 5% CO2.
2.2. Isolation, characterization and labelling of MSC-EVs
EVs were isolated from the supernatants of fifth-passage MSCs, as
previously described [15]. Briefly, the ratio of live and dead MSCs was
detected by using an automatic cell counter (Bodboge, Shenzhen, CHN),
the batch of which contained more than 99% live cells. The MSCs were
cultured in αMEM containing 10% EV-depleted FBS for 24 h, and then
the supernatants were harvested and processed via a series of centrifugation steps (300 ×g for 10 min, 2000 ×g for 10 min, and 10,000 ×g for
30 min; ST16R, Thermo Fisher, USA). Subsequently, the EVs were
collected via ultracentrifugation at 100,000 ×g for 70 min (XPN-100,
Beckman Coulter, USA). The nanoparticles were then characterized by
western blotting based on the positive markers TSG101 and CD9, as well
as negative marker calnexin, and examined via transmission electron
microscopy (TEM) and nanoparticle tracking analysis (NTA) to evaluate
morphology and size distribution, respectively. In addition, C5
Maleimide-Alexa 594 (CM-A954) (Invitrogen, A10256, California, USA)
was used to label MSC-EVs as our previous reports [17].
2.3. Macrophage activation and intervention
RAW 264.7 cells were pretreated with 10 μg / mL MSC-EVs
(Fig. S2A) for 12 h to ensure uptake, and then activated with 100 ng /
mL lipopolysaccharide (LPS; L2880, Sigma-Aldrich, CA, USA) for 12 h,
as previously reported [18]. ML385 (15 μM; HY-100523, Medchem
Express, New Jersey, USA), a pharmacological inhibitor of Nrf2, was
used to downregulate Nrf2 expression in RAW 264.7 cells.
2.4. Animal procedures
97 adult male (8–10-weeks old) C57BL/6 mice were purchased from
the Experimental Animal Center of Xi’an Jiaotong University, they were
housed in groups and were allowed a period to acclimatize to the laboratory environment before the start of the study. All animal procedures
were performed in accordance with the ARRIVE guidelines and
approved by the Ethics Review Board of Xi’an Central Hospital, Xi’an
Jiaotong University. Animals were housed under a controlled environment with a 12 / 12 h light / dark cycle with food and water provided.
Mice were intraperitoneally anesthetized using 4.0% chloralhydrate
(10 mL / kg) and administered LPS (10 mg / kg, diluted with saline)
intratracheally. A sham operation was performed in a similar manner
using saline solution (Sham group, n = 5). After LPS induction for 3 h,
50 μg MSC-EVs (diluted in 50 μL saline, Fig. S2B and C) and 50 μL saline
(vehicle) were administered via inhalation using an atomizer (YSKD BioTec, Beijing, China) as the ALI-inh + EVs (n = 20) and ALI-inh + Veh (n
= 15) groups, or administered by tail vein injection as the ALI-iv + EVs
(n = 15) and ALI-iv + Veh (n = 15) groups, respectively. The mice were
sacrificed at random via isoflurane at 24 h, 4 days (d), and 14 d after EV
administration, and lung tissues and blood samples were collected for
further analyses (Fig. S1). Also, to track the MSC-EVs in vitro, ALI mice
received MSC-EVs via inhalation (negative control, n = 3), or CM-A594
labeled MSC-EVs via inhalation (n = 3) and tail vein injection (n = 3) for
24 h, the lung tissues were then processed as above procedures. Additionally, 8 mice were excluded due to failed injection via tail vein, and
10 mice were died in the present experiments.
2.5. Luminex liquid chip
Following treatment with MSC-EVs for 24 h, whole blood was
collected from mouse orbits and centrifuged (10,000 rpm) for 10 min.
Supernatants from each group were assayed using a Luminex liquid chip
(Luminex 200, USA) for interleukin (IL)-1β, macrophage chemoattractant protein-1 (MCP-1), IL-1α, tumor necrosis factor (TNF)α, IL-12,
and IL-10 (MHSTCMAG-70 K, Mouse High Sensitivity T Cell Magnetic
相关实验
入的菌液易漏,后来尝试用50ul的加样器每次取20ul,采取多次注射的办法。做此类实验主要是注意感染的药物或液体不要漏出来,可以采取多次注射的办法。八、大鼠鼻腔给药1.有滴鼻和喷雾两种常见方式喷雾其实就是雾化吸入。滴鼻给药没有办法达到雾化吸入的效果。雾化吸入需要有雾化设备,一般医院的都有,但是医院的如果借不出来,自己家里的加湿器也可以凑合。雾化给药的时候,要把大鼠放在一个相对比较密闭的的容器中(当然要有透气孔),让大鼠尽可能多地接触药物,但是好象没有专门的这种容器,一般都是自制的,材料最好是有机
 技术资料
技术资料暂无技术资料 索取技术资料
文献支持
小鼠肺内雾化给药器,小鼠气管内雾化给药器
询价










