万千商家帮你免费找货
0 人在求购买到急需产品
- 详细信息
- 询价记录
- 文献和实验
- 技术资料
- 保存条件:
-20℃
- 保质期:
6个月
- 英文名:
pBT2
- 库存:
大量
- 供应商:
钰博生物
- 规格:
1ug/5ug
载体基本信息
| 出品公司: | Ybscience |
|---|---|
| 载体名称: | pBT2 |
| 质粒类型: | 金黄色葡萄球菌基因敲除载体,金葡菌基因敲除载体 |
| 高拷贝/低拷贝: | 低拷贝 |
| 克隆方法: | 限制性内切酶,多克隆位点 |
| 启动子: | -- |
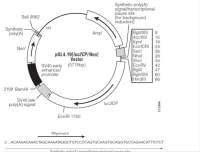
| 载体大小: | 6.97kb |
| 5' 测序引物及序列: | -- |
| 3' 测序引物及序列: | -- |
| 载体标签: | -- |
| 载体抗性: | 氨苄青霉素 |
| 筛选标记: | 氯霉素 |
| 克隆菌株: | DH5α 等 |
| 宿主细胞(系): | 金黄色葡萄球菌等革兰氏阳性菌 |
| 备注: | 该载体是大肠杆菌-金黄色葡萄球菌穿梭载体,低拷贝,在大肠中抗性为氨苄,在金葡菌中抗性为氯霉素。该质粒具有温度敏感性。 生物风对金黄色葡萄球菌的基因敲除具有丰富的实验经验,并且成功敲除过很多金黄色葡萄球菌基因,请有需要对金黄色葡萄球菌进行基因操作技术服务的老师和我们联系。 参考文献:Bruckner R: Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. FEMS microbiology letters 1997, 151(1):1-8. 相关的配套菌株为RN4220金黄色葡萄球菌, 相关的载体为pKOR1载体。 |
| 稳定性: | 稳表达 |
| 组成型/诱导型: | 组成型 |
| 病毒/非病毒: | 非病毒 |
载体质粒图谱和多克隆位点信息

载体简介
Protocols for gene deletion in Staphylococcus aureus Nov. 1, 2007
Preparation of competent Staphylococcus aureus cells
- 1. Remove Staphylococcus aureus cells from the vial with a sterile toothpick or inoculation loop, and streak it out on LB agar.
- 2. Incubate at 37°C overnight.
- 3. Pick a single colony and inoculate it in 5-10 ml of LB. Grow at 37°C overnight.
- 4. Add 1 ml overnight culture to 100 ml LB medium in a 500 ml flask, and shake at 37°C until an OD600 of 0.4 is reached (approximately 90–120 min).
- 5. Cool the culture on ice for 5 min, and transfer the culture to a sterile, round-bottom centrifuge tube.
- 6. Collect the cells by centrifugation at low speed (5-10 min, 2500 x g, 4°C).
- 7. Discard the supernatant carefully. Always keep the cells on ice.
- 8. Resuspend the cells gently in 0.5 M sucrose (10-15 ml for a 100 ml culture) at 4°C and keep the suspension on ice for additional 5 min.
- 9. Collect the cells by centrifugation (5 min, 2500 x g, 4°C).
- 10. Discard the supernatant carefully. Repeat step 8 and 9.
- 11. Resuspend the cells carefully in 1 ml ice-cold 0.5 M sucrose and keep the suspension on ice for 15 min.
- 12. Prepare aliquots of 100–200 μl in sterile microcentrifuge tubes and freeze in liquid nitrogen. Store the competent cells at –70°C.
Construction of deletion vector
- 1. PCR amplify a 400 bp fragment upstream and a 400 bp fragment downstream of the target gene.
- 2. PCR amplify the ermB (Em resistance marker) from pECI.
- 3. Digest the three fragments, ligate, and PCR amplify the ligated product.
- 4. Purify the PCR product, double digest it, and ligate it into pBT2.
- 5. Transform the ligated product into E. coli.
- 6. Pick clones that can grow on the LB plate containing Em (100 mg/ml), purify the plasmid and digest it.
- 7. If the result of enzyme digestion is correct, get further confirmation by sequencing.
Procedure for electroporation
- 1. Mix 500 ng of plasmid DNA with electrocompetent Staphylococcus aureus cells and place them in a Gene Pulser cuvette with a 0.2 cm electrode gap.
- 2. The settings for electroporation are as follows: Voltage, 2.5 kV; capacitor, 50 μF; resistance, 200 ohms.
- 3. After electroporation the cells are immediately placed in 400 μl of TSB with shaking (200-220 rpm, 37°C) for 1h. Plate the cells on Em-containing medium and incubate at 37°C.
Modify deletion vector
- 1. Before transform the plasmid into Staphylococcus aureus NCTC8325, the plasmid should be transformed into Staphylococcus aureus RN4220.
- 2. Pick clones, after overnight growth, extract the plasmid.
- 3. Then the plasmid is modified and can’t be digested by restriction enzyme system of NCTC8325.
Deletion of target gene
- 1. Extract plasmid from RN4220, transform it into NCTC8325.
- 2. Pick up clones, incubate in B-medium, 30°C, grow to late-stationary phase, then change temperature to 40°C, and grow overnight.
- 3. 1: 100 dilute the culture into fresh B-medium, and grow overnight.
- 4. Follow step 3, spread 1 μl overnight culture (diluted into 100 μl) on agar plate (containing Em 2.5 mg/ml). Screen clones which are Em-resistant and Cm-sensitive.
- 5. Repeat step 4 until Em-resistant, Cm-sensitive clones are found.
- 6. Extract genome DNA of these clones, use PCR for further check.
生物风对金黄色葡萄球菌的基因敲除具有丰富的实验经验,并且成功敲除过很多金黄色葡萄球菌基因,请有需要对金黄色葡萄球菌进行基因操作技术服务的老师和我们联系。
风险提示:丁香通仅作为第三方平台,为商家信息发布提供平台空间。用户咨询产品时请注意保护个人信息及财产安全,合理判断,谨慎选购商品,商家和用户对交易行为负责。对于医疗器械类产品,请先查证核实企业经营资质和医疗器械产品注册证情况。
- 作者
- 内容
- 询问日期
 文献和实验
文献和实验Burnell pBT载体多克隆位点酶切:见附件 由于酶切位点离得近,用NotI 和 XhoI 是否能同时进行双酶切,还是需要分部酶切? 另外,分别含 NotI和XhoI酶切位点的修饰引物一般需要几个保护碱基? Burnell 期待各位朋友为我答疑解惑,最近实验很郁闷,很悲剧! angler5156 不知道你用的什么公司的酶进行酶切
Non-fluorescent protein/RNA double-labeling
% hydrogen peroxide in PBTH. This will produce a brown signal. Stop the reaction with several rinses of PBTH. In situ HYBRIDIZATION : 1. Fix the embryos for 20 minutes with 5% formaldehyde in PBT (PBT= filtered 1 X PBS with 0.1% Tween-20
Non-fluorescent protein/RNA double-labeling
of PBTH. In situ HYBRIDIZATION : 1. Fix the embryos for 20 minutes with 5% formaldehyde in PBT (PBT= filtered 1 X PBS with 0.1% Tween-20) at room temperature. 2. Wash 3 X 5 minutes with PBT. 3. Incubate the embryos with 50 ug/ml
 技术资料
技术资料暂无技术资料 索取技术资料