相关产品推荐更多 >
万千商家帮你免费找货
0 人在求购买到急需产品
- 详细信息
- 文献和实验
- 技术资料
- 英文名:
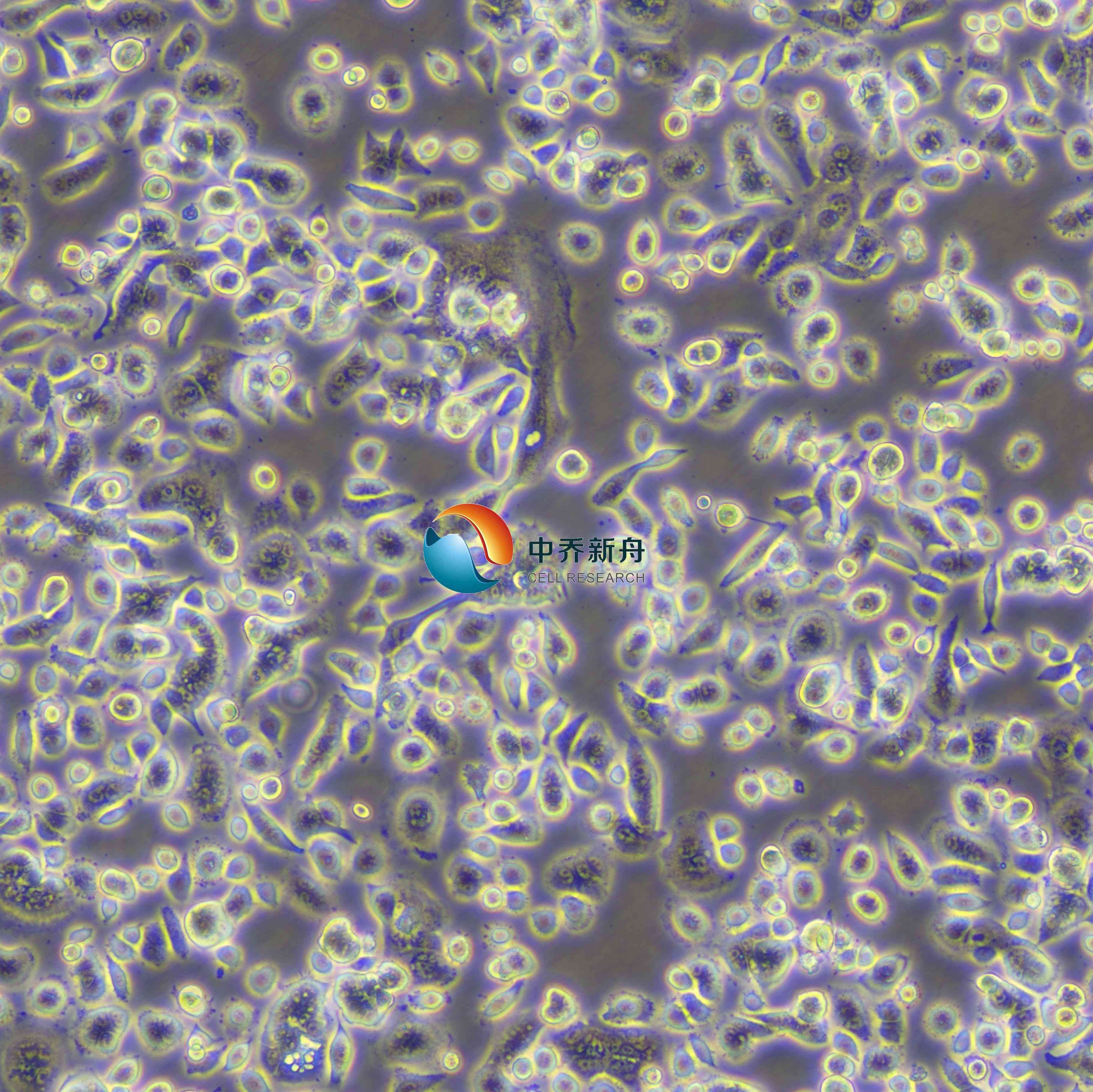
AsPC-1
- 库存:
大量
- 供应商:
中乔新舟
- 细胞类型:
细胞系
- 品系:
human
- 组织来源:
human
- 相关疾病:
否
- 物种来源:
human
- 免疫类型:
否
- 细胞形态:
咨询销售
- 器官来源:
human
- 运输方式:
T25瓶运输
- 年限:
5-10年
- 生长状态:
贴壁生长
- 规格:
5 x 10^5 cells/vial
|
产品名称 |
AsPC-1人转移胰腺癌细胞 |
|
货号 |
ZQ0254 |
|
产品介绍 |
一个胰腺癌病人的腹水中的细胞移植到裸鼠后建立了这个细胞株。 注意事项: 该细胞对血清质量敏感,建议用户使用优质血清进行培养。如遇细胞死亡较多或生长缓慢,可选用我司提供的该细胞完全培养液。 AsPC-1细胞生长呈不规则形态。细胞消化传代后细胞完全贴壁生长需要24-48小时,48小时才能换液操作。 |
|
种属 |
人 |
|
性别/年龄 |
女/62岁 |
|
组织 |
胰腺;来源于转移部位:腹水 |
|
疾病 |
腺癌 |
|
细胞类型 |
肿瘤细胞 |
|
形态学 |
上皮细胞 |
|
生长方式 |
贴壁 |
|
倍增时间 |
大约38~46小时 |
|
培养基和添加剂 |
RPMI-1640年(中乔新舟 货号:ZQ-200)+10%胎牛血清(中乔新舟货号:ZQ0500)+1%双抗(中乔新舟货号:CSP006) |
|
推荐完全培养基货号 |
ZM0254 |
|
生物安全等级 |
BSL-1 |
|
力量值位点信息 |
釉原蛋白:X CSF1PO: 10,13 D13S317: 9,12 D16S539: 11 D5S818: 12 D7S820: 12,13 TH01: 7,9.3 TPOX: 8,10 vWA: 17 |
|
培养条件 |
95%空气,5%二氧化碳;37℃ |
|
抗原表达/受体表达 |
|
|
基因表达 |
|
|
保藏机构 |
ATCC;BCRC CRL-1682;60494 ECACC;96020930 |
|
供应限制 |
仅供科研使用 |
上海中乔新舟生物科技有限公司成立于2011年,历经十多年发展,主要专注于细胞生物学产品的研究和开发,专注于为药企、各类科研机构及CRO企业提供符合标准规范的细胞培养服务、细胞培养基、细胞检测试剂盒、细胞培养试剂,胎牛血清和细胞生物学技术服务等。
公司一直致力于为高等院校、研究机构、医院、CRO及CDMO企业提供细胞培养完整解决方案,这些产品旨在满足细胞培养的多样需求,确保实验和研究的有效进行。引用中乔新舟(ZQXZBIO)产品和服务的文献超数千篇。

产品服务
细胞资源:原代细胞、细胞株、干细胞、示踪细胞、耐药株细胞、永生化细胞等基因工程细胞。
试剂产品:胎牛血清、完全培养基(适用于原代细胞及细胞株)、无血清培养基、基础培养基、细胞转染试剂、重组因子、胰酶和双抗等等细胞培养所有实验相关产品。
技术服务:稳转株构建、原代细胞分离、特殊培养基定制服务、细胞检测等。

目前产品已经畅销国内30多个省市,与客户建立长期的合作伙伴关系,共同实现成功。全体员工将不懈努力,继续为科研人员提供优良的产品和服务,致力成为全球细胞培养领域的参与者。
企业愿景
致力于成为国内细胞培养基产业的佼佼者,生物医药领域上游原材料的优良提供商。
企业使命
成长为专业细胞系及原代细胞培养供应商、专业细胞培养基及培养试剂生产商。
企业荣誉


风险提示:丁香通仅作为第三方平台,为商家信息发布提供平台空间。用户咨询产品时请注意保护个人信息及财产安全,合理判断,谨慎选购商品,商家和用户对交易行为负责。对于医疗器械类产品,请先查证核实企业经营资质和医疗器械产品注册证情况。
 文献和实验
文献和实验论文标题:亚微米级银颗粒是一种有前途的低毒性广谱强效抗癌治疗剂
DOI: 10.1002/adfm
发表时间: 2019-02-27
期刊:高级功能材料
影响因子: 15.621
货号:ZQ0254
产品名称:AsPC-1细胞
论文标题: NEAT1/miR-101-dependent Up-regulation of DNA-PKcs Enhances Malignant Behaviors of Pancreatic Ductal Adenocarcinoma Cells
DOI: 10.7150/jca.58824
发表时间: 2021-07-25
期刊: Journal of Cancer
影响因子: 4.207
货号: ZQ0254
产品名称: ASPC-1 cells
论文标题: The biological behavior of tRNA-derived fragment tRF-Leu-AAG in pancreatic cancer cells
DOI: 10.1080/21655979.2022.2064206
发表时间: 2022-04-20
期刊: Bioengineered
影响因子: 3.269
货号: ZQ0254
产品名称: ASPC-1 cells
论文标题: HOXB5 promotes proliferation, migration, and invasion of pancreatic cancer cell through the activation of the GSK3β/β-catenin pathway
DOI: 10.1097/CAD.0000000000000948
发表时间: 2020-09-01
期刊: ANTI-CANCER DRUGS
影响因子: 2.26
货号: ZQ0254
产品名称: ASPC-1 cells
PubMed=7182348; DOI=10.1007/BF02796382
Chen W.H., Horoszewicz J.S., Leong S.S., Shimano T., Penetrante R., Sanders W.H., Berjian R., Douglass H.O. Jr., Martin E.W., Chu T.M.
Human pancreatic adenocarcinoma: in vitro and in vivo morphology of a new tumor line established from ascites.
In Vitro 18:24-34(1982)
PubMed=6582512; DOI=10.1073/pnas.81.2.568
Mattes M.J., Cordon-Cardo C., Lewis J.L. Jr., Old L.J., Lloyd K.O.
Cell surface antigens of human ovarian and endometrial carcinoma defined by mouse monoclonal antibodies.
Proc. Natl. Acad. Sci. U.S.A. 81:568-572(1984)
PubMed=4023565
Tan M.H., Chu T.M.
Characterization of the tumorigenic and metastatic properties of a human pancreatic tumor cell line (AsPC-1) implanted orthotopically into nude mice.
Tumor Biol. 6:89-98(1985)
PubMed=1764370; DOI=10.1038/bjc.1991.467
Barton C.M., Staddon S.L., Hughes C.M., Hall P.A., O'Sullivan C., Kloppel G., Theis B., Russell R.C.G., Neoptolemos J., Williamson R.C.N., Lane D.P., Lemoine N.R.
Abnormalities of the p53 tumour suppressor gene in human pancreatic cancer.
Br. J. Cancer 64:1076-1082(1991)
PubMed=1630814
Ruggeri B., Zhang S.-Y., Caamano J., DiRado M., Flynn S.D., Klein-Szanto A.J.P.
Human pancreatic carcinomas and cell lines reveal frequent and multiple alterations in the p53 and Rb-1 tumor-suppressor genes.
Oncogene 7:1503-1511(1992)
PubMed=7972006; DOI=10.1073/pnas.91.23.11045
Okamoto A., Demetrick D.J., Spillare E.A., Hagiwara K., Hussain S.P., Bennett W.P., Forrester K., Gerwin B.I., Serrano M., Beach D.H., Harris C.C.
Mutations and altered expression of p16INK4 in human cancer.
Proc. Natl. Acad. Sci. U.S.A. 91:11045-11049(1994)
PubMed=8026879; DOI=10.1002/ijc.2910580207
Berrozpe G., Schaeffer J., Peinado M.A., Real F.X., Perucho M.
Comparative analysis of mutations in the p53 and K-ras genes in pancreatic cancer.
Int. J. Cancer 58:185-191(1994)
PubMed=8194712; DOI=10.1016/0016-5085(94)90422-7
Simon B., Weinel R., Hohne M., Watz J., Schmidt J., Kortner G., Arnold R.
Frequent alterations of the tumor suppressor genes p53 and DCC in human pancreatic carcinoma.
Gastroenterology 106:1645-1651(1994)
PubMed=8286197; DOI=10.1038/bjc.1994.24
Lohr J.-M., Trautmann B., Gottler M., Peters S., Zauner I., Maillet B., Kloppel G.
Human ductal adenocarcinomas of the pancreas express extracellular matrix proteins.
Br. J. Cancer 69:144-151(1994)
PubMed=21607521; DOI=10.3892/or.1.6.1223
Iguchi H., Morita R., Yasuda D., Takayanagi R., Ikeda Y., Takada Y., Shimazoe T., Nawata H., Kono A.
Alterations of the p53 tumor-suppressor gene and ki-ras oncogene in human pancreatic cancer-derived cell-lines with different metastatic potential.
Oncol. Rep. 1:1223-1227(1994)
PubMed=9331070
Teng D.H.-F., Perry W.L. III, Hogan J.K., Baumgard M.L., Bell R., Berry S., Davis T., Frank D., Frye C., Hattier T., Hu R., Jammulapati S., Janecki T., Leavitt A., Mitchell J.T., Pero R., Sexton D., Schroeder M., Su P.-H., Swedlund B., Kyriakis J.M., Avruch J., Bartel P., Wong A.K.C., Oliphant A., Thomas A., Skolnick M.H., Tavtigian S.V.
Human mitogen-activated protein kinase kinase 4 as a candidate tumor suppressor.
Cancer Res. 57:4177-4182(1997)
PubMed=9665481; DOI=10.1016/S0002-9440(10)65561-7
Paciucci R., Vila M.R., Adell T., Diaz V.M., Tora M., Nakamura T., Real F.X.
Activation of the urokinase plasminogen activator/urokinase plasminogen activator receptor system and redistribution of E-cadherin are associated with hepatocyte growth factor-induced motility of pancreas tumor cells overexpressing Met.
Am. J. Pathol. 153:201-212(1998)
PubMed=10027410; DOI=10.1016/S0002-9440(10)65298-4
Ghadimi B.M., Schrock E., Walker R.L., Wangsa D., Jauho A., Meltzer P.S., Ried T.
Specific chromosomal aberrations and amplification of the AIB1 nuclear receptor coactivator gene in pancreatic carcinomas.
Am. J. Pathol. 154:525-536(1999)
PubMed=10408907; DOI=10.1016/S0304-3835(98)00380-2
Bartsch D.K., Barth P., Bastian D., Ramaswamy A., Gerdes B., Chaloupka B., Deiss Y., Simon B., Schudy A.
Higher frequency of DPC4/Smad4 alterations in pancreatic cancer cell lines than in primary pancreatic adenocarcinomas.
Cancer Lett. 139:43-49(1999)
PubMed=11115575; DOI=10.3892/or.8.1.89
Sun C.-L., Yamato T., Furukawa T., Ohnishi Y., Kijima H., Horii A.
Characterization of the mutations of the K-ras, p53, p16, and SMAD4 genes in 15 human pancreatic cancer cell lines.
Oncol. Rep. 8:89-92(2001)
PubMed=11169959; DOI=10.1002/1097-0215(200002)9999:9999<::AID-IJC1049>3.0.CO;2-C
Sirivatanauksorn V., Sirivatanauksorn Y., Gorman P.A., Davidson J.M., Sheer D., Moore P.S., Scarpa A., Edwards P.A.W., Lemoine N.R.
Non-random chromosomal rearrangements in pancreatic cancer cell lines identified by spectral karyotyping.
Int. J. Cancer 91:350-358(2001)
PubMed=11787853; DOI=10.1007/s004280100474
Moore P.S., Sipos B., Orlandini S., Sorio C., Real F.X., Lemoine N.R., Gress T.M., Bassi C., Kloppel G., Kalthoff H., Ungefroren H., Lohr J.-M., Scarpa A.
Genetic profile of 22 pancreatic carcinoma cell lines. Analysis of K-ras, p53, p16 and DPC4/Smad4.
Virchows Arch. 439:798-802(2001)
PubMed=12692724; DOI=10.1007/s00428-003-0784-4
Sipos B., Moser S., Kalthoff H., Torok V., Lohr J.-M., Kloppel G.
A comprehensive characterization of pancreatic ductal carcinoma cell lines: towards the establishment of an in vitro research platform.
Virchows Arch. 442:444-452(2003)
PubMed=14695172
Iacobuzio-Donahue C.A., Ashfaq R., Maitra A., Adsay N.V., Shen-Ong G.L.-C., Berg K., Hollingsworth M.A., Cameron J.L., Yeo C.J., Kern S.E., Goggins M.G., Hruban R.H.
Highly expressed genes in pancreatic ductal adenocarcinomas: a comprehensive characterization and comparison of the transcription profiles obtained from three major technologies.
Cancer Res. 63:8614-8622(2003)
PubMed=15126341; DOI=10.1158/0008-5472.CAN-03-3159
Heidenblad M., Schoenmakers E.F.P.M., Jonson T., Gorunova L., Veltman J.A., van Kessel A.G., Hoglund M.
Genome-wide array-based comparative genomic hybridization reveals multiple amplification targets and novel homozygous deletions in pancreatic carcinoma cell lines.
Cancer Res. 64:3052-3059(2004)
PubMed=15367885; DOI=10.1097/00006676-200410000-00004
Loukopoulos P., Kanetaka K., Takamura M., Shibata T., Sakamoto M., Hirohashi S.
Orthotopic transplantation models of pancreatic adenocarcinoma derived from cell lines and primary tumors and displaying varying metastatic activity.
Pancreas 29:193-203(2004)
PubMed=15688027; DOI=10.1038/sj.onc.1208383
Heidenblad M., Lindgren D., Veltman J.A., Jonson T., Mahlamaki E.H., Gorunova L., van Kessel A.G., Schoenmakers E.F.P.M., Hoglund M.
Microarray analyses reveal strong influence of DNA copy number alterations on the transcriptional patterns in pancreatic cancer: implications for the interpretation of genomic amplifications.
Oncogene 24:1794-1801(2005)
PubMed=15770730; DOI=10.3748/wjg.v11.i10.1521
Ma J.-H., Patrut E., Schmidt J., Knaebel H.-P., Buchler M.W., Marten A.
Synergistic effects of interferon-alpha in combination with chemoradiation on human pancreatic adenocarcinoma.
World J. Gastroenterol. 11:1521-1528(2005)
PubMed=16912165; DOI=10.1158/0008-5472.CAN-06-0721
Calhoun E.S., Hucl T., Gallmeier E., West K.M., Arking D.E., Maitra A., Iacobuzio-Donahue C.A., Chakravarti A., Hruban R.H., Kern S.E.
Identifying allelic loss and homozygous deletions in pancreatic cancer without matched normals using high-density single-nucleotide polymorphism arrays.
Cancer Res. 66:7920-7928(2006)
PubMed=18298655; DOI=10.1111/j.1582-4934.2008.00289.x
Pilarsky C., Ammerpohl O., Sipos B., Dahl E., Hartmann A., Wellmann A., Braunschweig T., Lohr J.-M., Jesenofsky R., Friess H., Wente M.N., Kristiansen G., Jahnke B., Denz A., Ruckert F., Schackert H.K., Kloppel G., Kalthoff H., Saeger H.-D., Grutzmann R.
Activation of Wnt signalling in stroma from pancreatic cancer identified by gene expression profiling.
J. Cell. Mol. Med. 12:2823-2835(2008)
PubMed=18380791; DOI=10.1111/j.1349-7006.2008.00779.x
Suzuki A., Shibata T., Shimada Y., Murakami Y., Horii A., Shiratori K., Hirohashi S., Inazawa J., Imoto I.
Identification of SMURF1 as a possible target for 7q21.3-22.1 amplification detected in a pancreatic cancer cell line by in-house array-based comparative genomic hybridization.
Cancer Sci. 99:986-994(2008)
CLPUB00416
Oberlin L.
Treatment of pancreatic carcinoma cell lines in vitro and vivo with a monoclonal antibody against the transferrin receptor.
Thesis VMD (2009), Justus-Liebig-Universitat Giessen, Germany
DOI=10.4172/jpb.1000057
Yamada M., Fujii K., Koyama K., Hirohashi S., Kondo T.
The proteomic profile of pancreatic cancer cell lines corresponding to carcinogenesis and metastasis.
J. Proteomics Bioinformatics 2:1-18(2009)
PubMed=20164919; DOI=10.1038/nature08768
Bignell G.R., Greenman C.D., Davies H., Butler A.P., Edkins S., Andrews J.M., Buck G., Chen L., Beare D., Latimer C., Widaa S., Hinton J., Fahey C., Fu B.-Y., Swamy S., Dalgliesh G.L., Teh B.T., Deloukas P., Yang F.-T., Campbell P.J., Futreal P.A., Stratton M.R.
Signatures of mutation and selection in the cancer genome.
Nature 463:893-898(2010)
PubMed=20215515; DOI=10.1158/0008-5472.CAN-09-3458
Rothenberg S.M., Mohapatra G., Rivera M.N., Winokur D., Greninger P., Nitta M., Sadow P.M., Sooriyakumar G., Brannigan B.W., Ulman M.J., Perera R.M., Wang R., Tam A., Ma X.-J., Erlander M., Sgroi D.C., Rocco J.W., Lingen M.W., Cohen E.E.W., Louis D.N., Settleman J., Haber D.A.
A genome-wide screen for microdeletions reveals disruption of polarity complex genes in diverse human cancers.
Cancer Res. 70:2158-2164(2010)
PubMed=20418756; DOI=10.1097/MPA.0b013e3181c15963
Deer E.L., Gonzalez-Hernandez J., Coursen J.D., Shea J.E., Ngatia J., Scaife C.L., Firpo M.A., Mulvihill S.J.
Phenotype and genotype of pancreatic cancer cell lines.
Pancreas 39:425-435(2010)
成纤维细胞中过表达外泌体-miR- 520 后癌细胞功能及关键蛋白检测(建立胰腺癌细胞与成纤维细胞共培养体系,抑制共培养体系中外泌体分泌,过表达共培养体系中成纤维细胞 miR- 520b 来验证成纤维细胞 miR- 520b 能通过外泌体途径影响胰腺癌细胞功能。)miRNA- 520b 靶基因的确定(非重要结果,不出现在方法中)过表达外泌体 miRNA- 520b 能抑制肿瘤生长及转移能力(提取成纤维细胞外泌体,过表达外泌体中 miR- 520b 并与胰腺癌细胞共转染,将细胞接种于裸鼠中,观察裸鼠
为了逃避免疫攻击,癌细胞又出新招!Cancer Cell 发现连胶原蛋白也「叛变」了……
binds to a3b1 integrin and affects tumor microbiome and immunity to promote pancreatic cancer 的研究性论文,发现胰腺癌细胞产生的 I 型胶原(Col1)蛋白是一种异常的同源三聚体变体,具有致癌特性。癌细胞中 Col1 同源三聚体的缺失可抑制肿瘤进展并重塑肿瘤微生物组,增强 T 细胞浸润,使抗 PD-1 免疫治疗更有效。 图片来源:Cancer Cell 研究内容 胰腺癌细胞中存在特异性胶原
益生菌竟然有害健康?破坏免疫抑制,刺激肿瘤生长,化身「癌症帮凶」
抑制剂 CH223191 进一步验证其对免疫浸润表型和肿瘤生长的影响,CH223191 能够增加 PDL1 抗体的抗肿瘤效果,二者联用能够有效地改善小鼠的生存状况。这些数据证明 AhR 能够抑制 T 细胞成熟,从而破坏胰腺癌细胞的免疫抑制。 图片来源:Immunity AhR 改变细胞基因转录 通过质谱流式细胞技术(CyTOF)和单细胞测序技术,研究人员分别在肿瘤组织中鉴定出三组巨噬细胞簇。iGSEA 分析这三组巨噬细胞的基因富集情况,结果表明 AhR 能够影响包括 IFNg, IRF
 技术资料
技术资料暂无技术资料 索取技术资料