相关产品推荐更多 >
万千商家帮你免费找货
0 人在求购买到急需产品
- 详细信息
- 文献和实验
- 技术资料
- 规格:
1L
-
产品描述(Product Details)
CelThera™ GMP T Cell Expansion Medium is a serum-free culture medium specifically developed to support human T cell culture. It is a serum-free, animal origin-free T cell maintenance and expansion medium produced under GMP conditions.
Compared to traditional culture media or xeno-free culture media, animal origin-free culture media can better reduce the risk of introducing potential pathogenic microorganisms during culture process, improve batch-to-batch consistency, and prevent T cell overactivation by undefined components in the serum.
CelThera™ GMP T Cell Expansion Medium does not require the addition of any serum or serum replacements and also maintains the high fold expansion of T cells. If users choose to add serum or serum replacement, the dosage should be determined by specific T cell applications.
-
优势特色(Features)
- Serum-free, animal origin-free (AOF), and exogenous growth factors free.
- Designed to support low-density seeding and high fold expansion of T cells.
- Suitable for large-scale T cell expansion.
- No additional serum or serum replacements needed.
- T cell phenotypes similar to media supplemented with serum or serum replacement.
- Contains only recombinant proteins as components, and no antibiotics included in formulation.
- Produced according to current GMP guidelines.
-
存储(Storage)
The CelThera™ GMP T Cell Expansion Medium is stable for 18 months when stored under 2-8°C, protect from light.
The CelThera™ GMP T Cell Expansion Supplement is stable for 12 months when stored under -20°C or below, protect from light.
-
应用数据(Application Data)
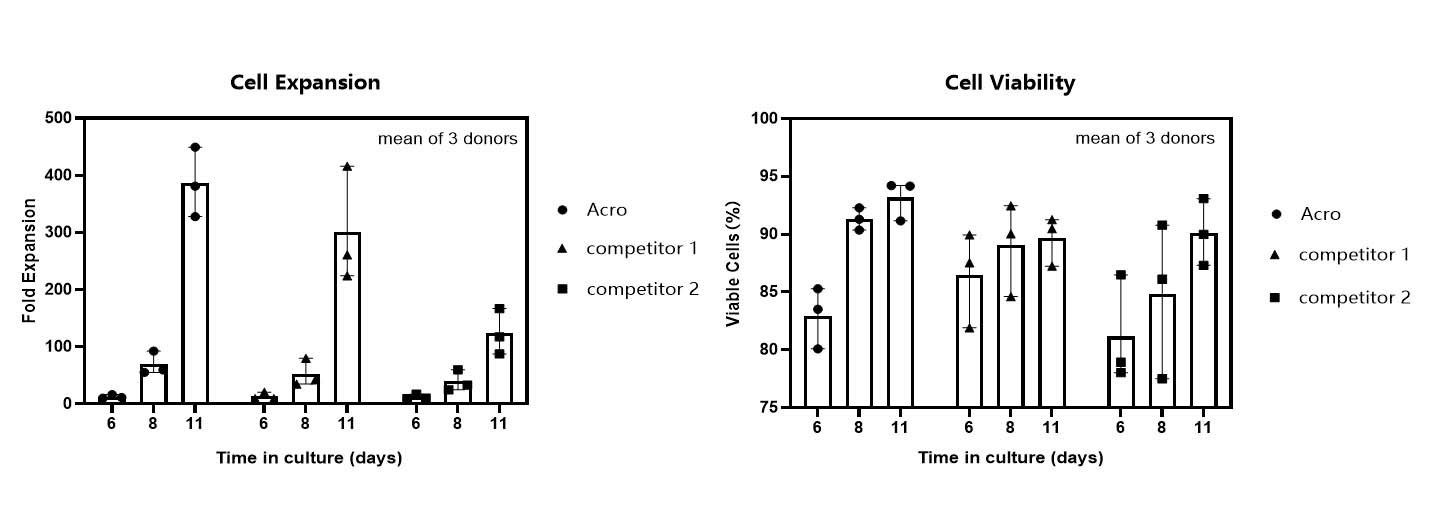
T cell expansion rate and cell viability in various media.
T cells from PBMCs of 3 different donors were activated and cultured for 11 days in various media supplemented with 300 IU/ml Acro GMP IL-2 (Cat. No. GMP-L02H14). Cell count and viability were performed on day 6, day 8 and day 11 by trypan blue staining. It indicated that cells in Acro T cell medium (Cat. No. GMP-CM3101) had a faster proliferation rate and higher viability than that of the other two media. -
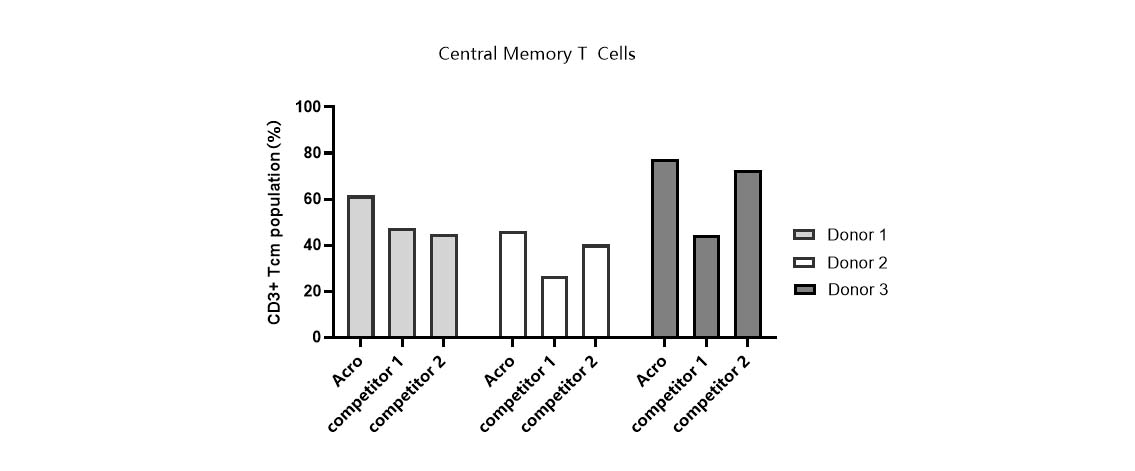
Tcm ratio in various media.
T cells from PBMCs of 3 different donors were activated and cultured in various media supplemented with 300 IU/ml Acro GMP IL-2 (Cat. No. GMP-L02H14). Tcm percentage (CD45RO+/CCR7+) was determined by flow cytometry when cells reached about 50-fold expansion. It indicated that cells in Acro T cell medium (Cat. No. GMP-CM3101) had a higher percentage of central memory T cells than that of the other two media. -
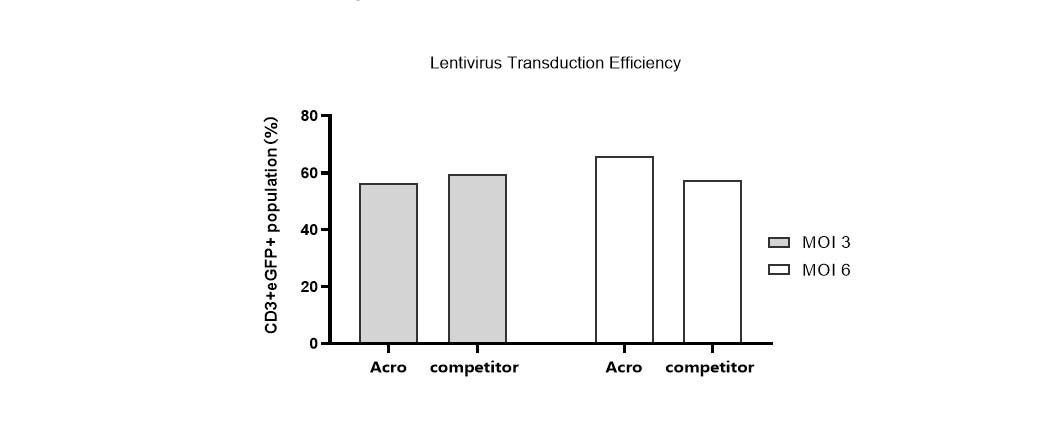
Lentivirus transduction efficiency in various media.
T cells from PBMCs were activated and cultured in various media supplemented with 300 IU/ml Acro GMP IL-2 (Cat. No. GMP-L02H14). 24 hours after activation, the cells were transduced with pLenti-CMV-EGFP-puro lentivirus (MOI=3 or 6). 24hrs after transduction, the lentivirus was removed by centrifugation. Then, the cells were cultured for 48hrs and the CD3+eGFP+ population was detected by flow cytometry. It indicated that cells in Acro T cell medium (Cat. No. GMP-CM3101) had a similar lentiviral transduction efficiency to that of the other medium.
-
多供体验证数据(Multiple Donor Verification)
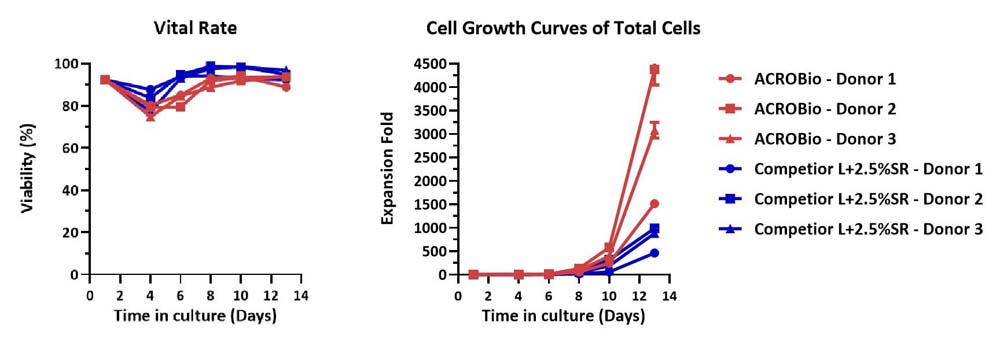
Human PBMCs were cultured with GMP Human IL-2 Protein (ACROBiosystems, Cat. No. GMP-L02H14) with CelThera™ GMP T Cell Expansion Medium (ACROBiosystems, Cat. No. GMP-CM3101) or T cell culture medium (Competitor L +2.5% SR) for two weeks. The result shows that CelThera™ GMP T Cell Expansion Medium (ACROBiosystems) can be comparable to Competitor L +2.5% SR. Notably, the cells exhibit better expansion in CelThera™ GMP T Cell Expansion Medium (ACROBiosystems, Cat. No. GMP-CM3101).
-
大规模培养数据(Large-scale Culture Verification)
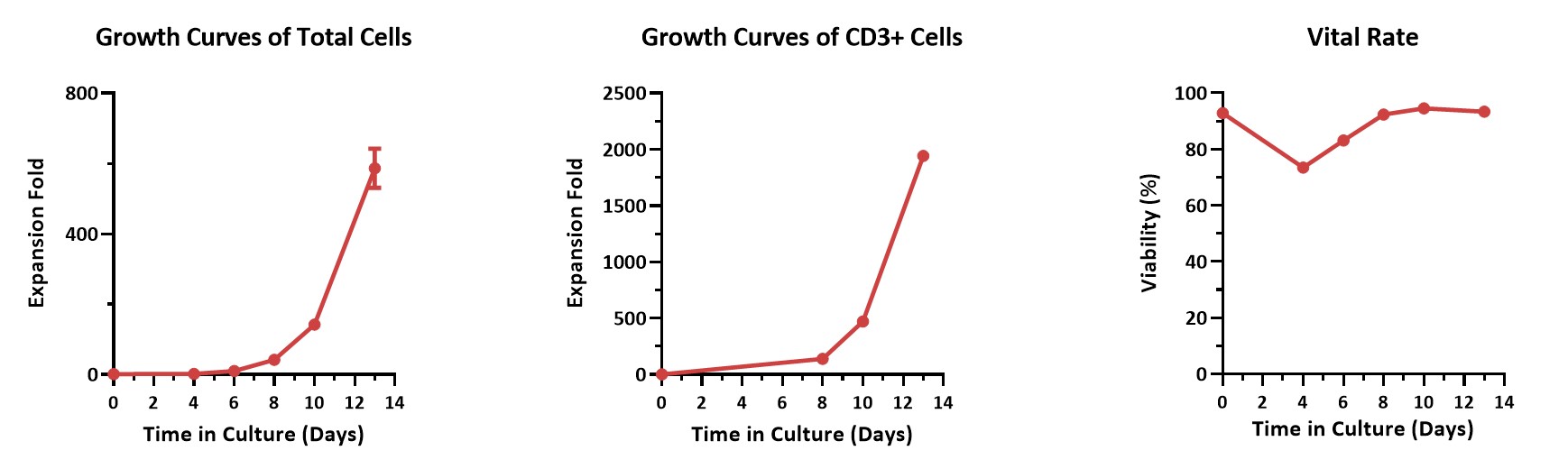
Human PBMCs were activated using 0.2 µg/mL GMP Monoclonal Anti-Human CD3 Antibody (OKT3) (ACROBiosystems, Cat. No. GMP-MC0323) and 1 µg/mL GMP Monoclonal Anti-Human CD28 Antibody (ACROBiosystems, Cat. No. GMP-MC2824), cultured with CelThera™ GMP T Cell Expansion culture medium (ACROBiosystems, Cat. No. GMP-CM3101) supplemented with 500 IU/mL GMP Human IL-2 Protein (ACROBiosystems, Cat. No. GMP-L02H14) for two weeks. The results showed that GMP human IL-2 protein, GMP monoclonal anti-human CD3 antibody (OKT3), GMP monoclonal anti-human CD28 antibody, and CelThera™ GMP T cell expansion medium could be used to culture T cells in a 3L large system. It can efficiently expand cells with high viability.
风险提示:丁香通仅作为第三方平台,为商家信息发布提供平台空间。用户咨询产品时请注意保护个人信息及财产安全,合理判断,谨慎选购商品,商家和用户对交易行为负责。对于医疗器械类产品,请先查证核实企业经营资质和医疗器械产品注册证情况。
 文献和实验
文献和实验GE推出首个符合GMP制备单核细胞培养基Ficoll-Paque™ PREMIUM
相关专题 细胞培养基的配制 GE医疗集团于10月17号推出Ficoll-Paque™ PREMIUM产品。Ficoll-Paque是已经有30年历史的,用于从血液和骨髓中制备人类单核细胞 的产品。Ficoll-Paque PREMIUM比起它之前的Ficoll-Paque PLUS除了证明有同样可信的结果外,还采用了ISO质量系统和消毒生产标准。 之前的Ficoll-Paque PLUS作为一种消毒后的培养基在利用骨髓
。细胞数量、培养基体积、多聚体用量配比参考以下表格: 培养板 96 孔板 48 孔板 24 孔板 T 细胞数量 5-8×10^4 2-5×10^4 0.5-1×10^6 培养基体积(ml) 0.1-0.2 0.5-1 1-2 CD3/CD28 Streptamer premix(μl) 3 15 30 3. 培养过程中,每天观察
人 iPS 细胞体外分化为气道上皮肺类器官用于呼吸系统疾病研究应用以及iPSC操作指南
(SCM075) 添加到 7-10 mL 人 ES/iPS 细胞扩增培养基 (SCM130) 中,最终浓度为 10μM。 2. 用 ECM 凝胶 (CC131) 涂层 6 孔板。 3. 吸出培养基。用 2 mL 的 DMEM/F12 或 1X PBS 清洗孔。吸出 1 mL Accumax™ 溶液 (A7089) 并将其添加到孔中。在 37°C下孵育 5-6 分钟。手掌敲击板,以帮助解离细胞。 4. 向孔中加入 1 mL 单细胞传代培养基(来自步骤 1)。用 5 mL 移液器上下吹打 1-3 次以分离
 技术资料
技术资料暂无技术资料 索取技术资料











