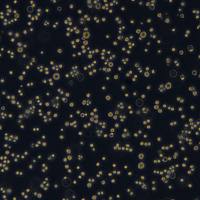
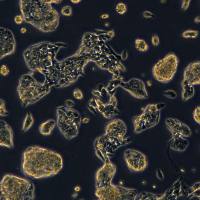
PANC10.05细胞系
- ¥1800
- EK-Bioscience
- MY-Y1745
- 2025年07月09日
相关产品推荐更多 >
万千商家帮你免费找货
0 人在求购买到急需产品
- 详细信息
- 文献和实验
- 技术资料
- 规格:
T25
Panc10.05/Panc10.05细胞系/Panc10.05细胞株/Panc10.05胰腺腺癌细胞
Cell line name Panc 10.05
Synonyms Panc-10.05; Panc10.05; PANC-10-05; PANC 1005; PANC1005; Panc1005; Pa16C; PL12; PL-12
Accession CVCL_1639
Secondary accession CVCL_H600
Resource Identification Initiative To cite this cell line use: Panc 10.05 (RRID:CVCL_1639)
Comments Part of: Cancer Dependency Map project (DepMap) (includes Cancer Cell Line Encyclopedia - CCLE).
Part of: COSMIC cell lines project.
Part of: MD Anderson Cell Lines Project.
Part of: NCI RAS program mutant KRAS cell line panel.
Part of: RAS genetic alteration cell panel (ATCC TCP-1031).
From: Johns Hopkins University School of Medicine; Baltimore; USA.
Population: Caucasian.
Doubling time: 19.2 hours (PubMed=9612602); 30 hours (PubMed=25984343).
Microsatellite instability: Stable (MSS) (Sanger).
Omics: CRISPR phenotypic screen.
Omics: Deep exome analysis.
Omics: Deep proteome analysis.
Omics: Deep quantitative proteome analysis.
Omics: DNA methylation analysis.
Omics: Metabolome analysis.
Omics: Protein expression by reverse-phase protein arrays.
Omics: shRNA library screening.
Omics: SNP array analysis.
Omics: Transcriptome analysis by microarray.
Omics: Transcriptome analysis by RNAseq.
Derived from site: In situ; Pancreas; UBERON=UBERON_0001264.
PubMed=26216984; DOI=10.1073/pnas.1501605112; PMCID=PMC4538616
Daemen A., Peterson D., Sahu N., McCord R., Du X.-N., Liu B., Kowanetz K., Hong R., Moffat J., Gao M., Boudreau A., Mroue R., Corson L., O'Brien T., Qing J., Sampath D., Merchant M., Yauch R.L., Manning G., Settleman J., Hatzivassiliou G., Evangelista M.
Metabolite profiling stratifies pancreatic ductal adenocarcinomas into subtypes with distinct sensitivities to metabolic inhibitors.
Proc. Natl. Acad. Sci. U.S.A. 112:E4410-E4417(2015)
PubMed=27259358; DOI=10.1074/mcp.M116.058313; PMCID=PMC4974343
Humphrey E.S., Su S.-P., Nagrial A.M., Hochgrafe F., Pajic M., Lehrbach G.M., Parton R.G., Yap A.S., Horvath L.G., Chang D.K., Biankin A.V., Wu J.-M., Daly R.J.
Resolution of novel pancreatic ductal adenocarcinoma subtypes by global phosphotyrosine profiling.
Mol. Cell. Proteomics 15:2671-2685(2016)
PubMed=27397505; DOI=10.1016/j.cell.2016.06.017; PMCID=PMC4967469
Iorio F., Knijnenburg T.A., Vis D.J., Bignell G.R., Menden M.P., Schubert M., Aben N., Goncalves E., Barthorpe S., Lightfoot H., Cokelaer T., Greninger P., van Dyk E., Chang H., de Silva H., Heyn H., Deng X.-M., Egan R.K., Liu Q.-S., MT., Mitropoulos X., Richardson L., Wang J.-H., Zhang T.-H., Moran S., Sayols S., Soleimani M., Tamborero D., Lopez-Bigas N., Ross-Macdonald P., Esteller M., Gray N.S., Haber D.A., Stratton M.R., Benes C.H., Wessels L.F.A., Saez-Rodriguez J., McDermott U., Garnett M.J.
A landscape of pharmacogenomic interactions in cancer.
Cell 166:740-754(2016)
PubMed=28196595; DOI=10.1016/j.ccell.2017.01.005; PMCID=PMC5501076
Li J., Zhao W., Akbani R., Liu W.-B., Ju Z.-L., Ling S.-Y., Vellano C.P., Roebuck P., Yu Q.-H., Eterovic A.K., Byers L.A., Davies M.A., Deng W.-L., Gopal Y.N.V., Chen G., von Euw E.M., Slamon D.J., Conklin D., Heymach J.V., Gazdar A.F., Minna J.D., Myers J.N., Lu Y.-L., Mills G.B., Liang H.
Characterization of human cancer cell lines by reverse-phase protein arrays.
Cancer Cell 31:225-239(2017)
PubMed=29444439; DOI=10.1016/j.celrep.2018.01.051; PMCID=PMC6343826
Yuan T.L., Amzallag A., Bagni R., Yi M., Afghani S., Burgan W., Fer N., Strathern L.A., Powell K., Smith B., Waters A.M., Drubin D.A., Thomson T., Liao R., Greninger P., Stein G.T., Murchie E., Cortez E., Egan R.K., Procter L., Bess M., Cheng K.T., Lee C.-S., Lee L.C., Fellmann C., Stephens R., Luo J., Lowe S.W., Benes C.H., McCormick F.
Differential effector engagement by oncogenic KRAS.
Cell Rep. 22:1889-1902(2018)
PubMed=30894373; DOI=10.1158/0008-5472.CAN-18-2747; PMCID=PMC6445675
Dutil J., Chen Z.-H., Monteiro A.N.A., Teer J.K., Eschrich S.A.
An interactive resource to probe genetic diversity and estimated ancestry in cancer cell lines.
Cancer Res. 79:1263-1273(2019)
PubMed=30971826; DOI=10.1038/s41586-019-1103-9
Behan F.M., Iorio F., Picco G., Goncalves E., Beaver C.M., Migliardi G., Santos R., Rao Y., Sassi F., Pinnelli M., Ansari R., Harper S., Jackson D.A., McRae R., Pooley R., Wilkinson P., van der Meer D.J., Dow D., Buser-Doepner C.A., Bertotti A., Trusolino L., Stronach E.A., Saez-Rodriguez J., Yusa K., Garnett M.J.
Prioritization of cancer therapeutic targets using CRISPR-Cas9 screens.
Nature 568:511-516(2019)
PubMed=31068700; DOI=10.1038/s41586-019-1186-3; PMCID=PMC6697103
Ghandi M., Huang F.W., Jane-Valbuena J., Kryukov G.V., Lo C.C., McDonald E.R. 3rd, Barretina J.G., Gelfand E.T., Bielski C.M., Li H.-X., Hu K., Andreev-Drakhlin A.Y., Kim J., Hess J.M., Haas B.J., Aguet F., Weir B.A., Rothberg M.V., Paolella B.R., Lawrence M.S., Akbani R., Lu Y.-L., Tiv H.L., Gokhale P.C., de Weck A., Mansour A.A., Oh C., Shih J., Hadi K., Rosen Y., Bistline J., Venkatesan K., Reddy A., Sonkin D., Liu M., Lehar J., Korn J.M., Porter D.A., Jones M.D., Golji J., Caponigro G., Taylor J.E., Dunning C.M., Creech A.L., Warren A.C., McFarland J.M., Zamanighomi M., Kauffmann A., Stransky N., Imielinski M., Maruvka Y.E., Cherniack A.D., Tsherniak A., Vazquez F., Jaffe J.D., Lane A.A., Weinstock D.M., Johannessen C.M., Morrissey M.P., Stegmeier F., Schlegel R., Hahn W.C., Getz G., Mills G.B., Boehm J.S., Golub T.R., Garraway L.A., Sellers W.R.
Next-generation characterization of the Cancer Cell Line Encyclopedia.
Nature 569:503-508(2019)
PubMed=35839778; DOI=10.1016/j.ccell.2022.06.010; PMCID=PMC9387775
Goncalves E., Poulos R.C., Cai Z.-X., Barthorpe S., Manda S.S., Lucas N., Beck A., Bucio-Noble D., Dausmann M., Hall C., Hecker M., Koh J., Lightfoot H., Mahboob S., Mali I., Morris J., Richardson L., Seneviratne A.J., Shepherd R., Sykes E., Thomas F., Valentini S., Williams S.G., Wu Y.-X., Xavier D., MacKenzie K.L., Hains P.G., Tully B., Robinson P.J., Zhong Q., Garnett M.J., Reddel R.R.
Pan-cancer proteomic map of 949 human cell lines.
Cancer Cell 40:835-849.e8(2022)
风险提示:丁香通仅作为第三方平台,为商家信息发布提供平台空间。用户咨询产品时请注意保护个人信息及财产安全,合理判断,谨慎选购商品,商家和用户对交易行为负责。对于医疗器械类产品,请先查证核实企业经营资质和医疗器械产品注册证情况。
 文献和实验
文献和实验*发表【中文论文】请标注:由上海酶研生物科技有限公司提供;
*发表【英文论文】请标注:From Shanghai EK-Bioscience Biotechnology Co., Ltd.
0.05mol/L Tris-HCl缓冲液(pH7.19~9.10)
12.0 18.0 8.05 10.0 11.0
straightforward assayprotocols using immunoblotting. 2. Materials 2.1. Cell Culture and Lysis 1. MiaPaCa2 and Panc04.03 pancreatic cancer cells are maintainedin DMEM and RPMI-1640 (Gibco/BRL, Bethesda, MD),respectively, supplemented with 10% fetal bovine
一、原理用Na2 51 CrO 4 标记靶细胞,若待检效应细胞能杀伤靶细胞,则51 Cr( 铬) 从靶细胞内释出。以γ计数仪测定释出的51 Cr 放射活性,靶细胞溶解破坏越多,51 Cr 释放就越多,上清液的放射活性也就越高,应用公式可计算出待检效应细胞的杀伤活性。二、步骤(以CTL杀伤肿瘤细胞为例):1、效应细胞将分离的外周血T细胞, 培养7d后按照DC:T = 1:10的比例分别加入自身细胞致敏DC,细胞系致敏DC及未致敏DC, 继续培养3-4d后收集T细胞, 此即为效应细胞CTL.2
 技术资料
技术资料暂无技术资料 索取技术资料