相关产品推荐更多 >
万千商家帮你免费找货
0 人在求购买到急需产品
- 详细信息
- 文献和实验
- 技术资料
- 英文名:
Caki-2
- 库存:
100
- 供应商:
中乔新舟
- 品系:
细胞系
- 运输方式:
常温
- 年限:
液氮长期
- 生长状态:
贴壁生长
- 规格:
T25
|
产品名称 |
Caki-2人乳头状肾细胞癌细胞 |
|
货号 |
ZQ0844 |
|
产品介绍 |
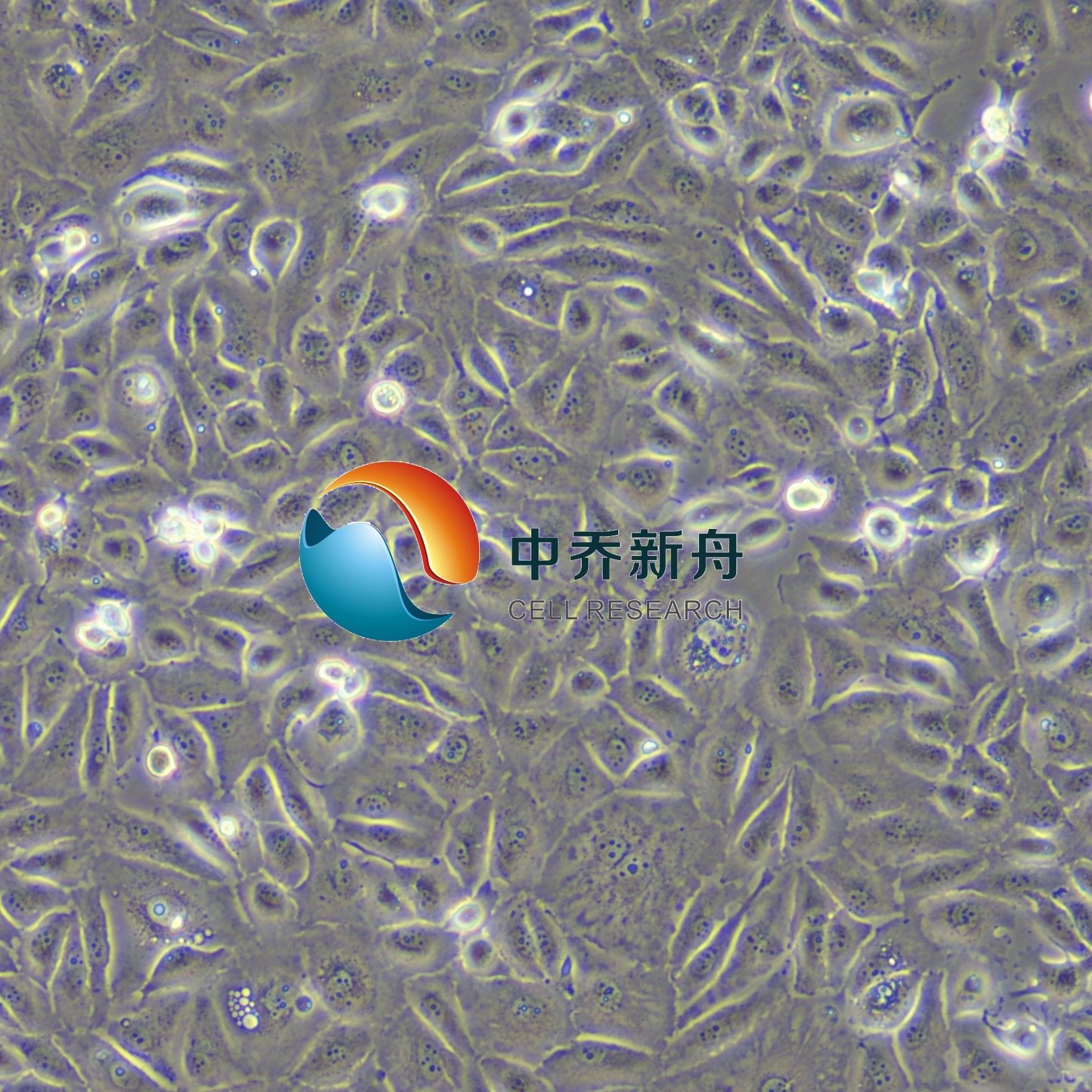
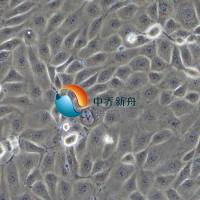
Caki-2 是一种人透明细胞肾细胞癌 (ccRCC) 细胞系,它源自一位69岁白人男性的初期肾腺癌组织。在体外培养条件下表现出上皮形态并粘附。它是研究肾癌机制和治疗反应的重要临床前模型。Caki-2 细胞系尤其值得注意的是它对某些化疗药物具有耐药性;与 Caki-1 细胞系相比,它对 5-氟尿嘧啶和多激酶抑制剂索拉非尼(靶向 VEGFRs 1-3、PDGFR-b 和 Raf-1)的敏感性降低。这种差异敏感性对于研究药物耐药机制和评估肾细胞癌的新治疗策略具有重要意义。
|
|
种属 |
人 |
|
性别/年龄 |
男/69岁 |
|
组织 |
肾脏 |
|
疾病 |
乳头状肾细胞癌 |
|
细胞类型 |
肿瘤细胞 |
|
形态学 |
上皮的 |
|
生长方式 |
贴壁 |
|
倍增时间 |
~30-40 hours (DSMZ=ACC-54) |
|
培养基和添加剂 |
McCoy's 5A 基础培养基(中乔新舟 货号:ZQ-1000)+10%胎牛血清(中乔新舟 货号:ZQ500-A)+1%P/S(中乔新舟 货号:CSP006) |
|
推荐完全培养基货号 |
ZM0844 |
|
生物安全等级 |
BSL-1 |
|
培养条件 |
95%空气,5%二氧化碳;37℃ |
|
STR位点信息 |
Amelogenin: X,Y CSF1PO: 10,12 D2S1338: 17,20 D3S1358: 14 D5S818: 11 D7S820: 12 D8S1179: 10 D13S317: 10 D16S539 :9,13 D18S51: 17 D19S433: 13,14 D21S11: 27,31 FGA: 22 Penta D: 10,13 Penta E: 7,17 TH01: 6 TPOX :9,11 vWA :16,17 |
|
抗原表达/受体表达 |
*** |
|
基因表达 |
*** |
|
保藏机构 |
ATCC; HTB-47 DSMZ; ACC-54 ECACC; 93120819 |
|
供应限制 |
仅供科研使用 |
上海中乔新舟生物科技有限公司成立于2011年,历经十多年发展,主要专注于细胞生物学产品的研究和开发,专注于为药企、各类科研机构及CRO企业提供符合标准规范的细胞培养服务、细胞培养基、细胞检测试剂盒、细胞培养试剂,胎牛血清和细胞生物学技术服务等。
公司一直致力于为高等院校、研究机构、医院、CRO及CDMO企业提供细胞培养完整解决方案,这些产品旨在满足细胞培养的多样需求,确保实验和研究的有效进行。引用中乔新舟(ZQXZBIO)产品和服务的文献超数千篇。

产品服务
细胞资源:原代细胞、细胞株、干细胞、示踪细胞、耐药株细胞、永生化细胞等基因工程细胞。
试剂产品:胎牛血清、完全培养基(适用于原代细胞及细胞株)、无血清培养基、基础培养基、细胞转染试剂、重组因子、胰酶和双抗等等细胞培养所有实验相关产品。
技术服务:稳转株构建、原代细胞分离、特殊培养基定制服务、细胞检测等。

目前产品已经畅销国内30多个省市,与客户建立长期的合作伙伴关系,共同实现成功。全体员工将不懈努力,继续为科研人员提供优良的产品和服务,致力成为全球细胞培养领域的参与者。
企业愿景
致力于成为国内细胞培养基产业的佼佼者,生物医药领域上游原材料的优良提供商。
企业使命
成长为专业细胞系及原代细胞培养供应商、专业细胞培养基及培养试剂生产商。
企业荣誉


风险提示:丁香通仅作为第三方平台,为商家信息发布提供平台空间。用户咨询产品时请注意保护个人信息及财产安全,合理判断,谨慎选购商品,商家和用户对交易行为负责。对于医疗器械类产品,请先查证核实企业经营资质和医疗器械产品注册证情况。
 文献和实验
文献和实验
DOI=10.1007/978-1-4757-1647-4_5
Fogh J., Trempe G.L.
New human tumor cell lines.
(In) Human tumor cells in vitro; Fogh J. (eds.); pp.115-159; Springer; New York (1975)
PubMed=327080; DOI=10.1093/jnci/59.1.221
Fogh J., Fogh J.M., Orfeo T.
One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice.
J. Natl. Cancer Inst. 59:221-226(1977)
PubMed=833871; DOI=10.1093/jnci/58.2.209
Fogh J., Wright W.C., Loveless J.D.
Absence of HeLa cell contamination in 169 cell lines derived from human tumors.
J. Natl. Cancer Inst. 58:209-214(1977)
PubMed=571047
Fogh J.
Cultivation, characterization, and identification of human tumor cells with emphasis on kidney, testis, and bladder tumors.
Natl. Cancer Inst. Monogr. 49:5-9(1978)
PubMed=6244232
Williams R.D.
Human urologic cancer cell lines.
Invest. Urol. 17:359-363(1980)
PubMed=6935474; DOI=10.1093/jnci/66.2.239
Wright W.C., Daniels W.P., Fogh J.
Distinction of seventy-one cultured human tumor cell lines by polymorphic enzyme analysis.
J. Natl. Cancer Inst. 66:239-247(1981)
PubMed=7459858
Rousset M., Zweibaum A., Fogh J.
Presence of glycogen and growth-related variations in 58 cultured human tumor cell lines of various tissue origins.
Cancer Res. 41:1165-1170(1981)
PubMed=6582512; DOI=10.1073/pnas.81.2.568
Mattes M.J., Cordon-Cardo C., Lewis J.L. Jr., Old L.J., Lloyd K.O.
Cell surface antigens of human ovarian and endometrial carcinoma defined by mouse monoclonal antibodies.
Proc. Natl. Acad. Sci. U.S.A. 81:568-572(1984)
PubMed=3518877; DOI=10.3109/07357908609038260
Fogh J.
Human tumor lines for cancer research.
Cancer Invest. 4:157-184(1986)
PubMed=7591954; DOI=10.1111/j.1349-7006.1995.tb03087.x
Kinoshita H., Yamada H., Ogawa O., Kakehi Y., Osaka M., Nakamura E., Mishina M., Habuchi T., Takahashi R., Sugiyama T., Yoshida O.
Contribution of chromosome 9p21-22 deletion to the progression of human renal cell carcinoma.
Jpn. J. Cancer Res. 86:795-799(1995)
PubMed=10723130; DOI=10.1038/sj.onc.1203449
Alimov A., Kost-Alimova M., Liu J., Li C.-D., Bergerheim U.S.R., Imreh S., Klein G., Zabarovsky E.R.
Combined LOH/CGH analysis proves the existence of interstitial 3p deletions in renal cell carcinoma.
Oncogene 19:1392-1399(2000)
PubMed=10929426; DOI=10.1007/s002400000103
Shintaku I., Kawagoe N., Yutani S., Hoshi S., Orikasa S., Yoshizumi O., Itoh K.
Expression of the SART1 tumor rejection antigen in renal cell carcinoma.
Urol. Res. 28:178-184(2000)
PubMed=15585611; DOI=10.1158/1078-0432.CCR-04-0072
Tykodi S.S., Warren E.H., Thompson J.A., Riddell S.R., Childs R.W., Otterud B.E., Leppert M.F., Storb R., Sandmaier B.M.
Allogeneic hematopoietic cell transplantation for metastatic renal cell carcinoma after nonmyeloablative conditioning: toxicity, clinical response, and immunological response to minor histocompatibility antigens.
Clin. Cancer Res. 10:7799-7811(2004)
PubMed=17409424; DOI=10.1158/0008-5472.CAN-06-4571
Furge K.A., Chen J.-D., Koeman J., Swiatek P.J., Dykema K., Lucin K., Kahnoski R., Yang X.-M.J., Teh B.T.
Detection of DNA copy number changes and oncogenic signaling abnormalities from gene expression data reveals MYC activation in high-grade papillary renal cell carcinoma.
Cancer Res. 67:3171-3176(2007)
PubMed=20215515; DOI=10.1158/0008-5472.CAN-09-3458
Rothenberg S.M., Mohapatra G., Rivera M.N., Winokur D., Greninger P., Nitta M., Sadow P.M., Sooriyakumar G., Brannigan B.W., Ulman M.J., Perera R.M., Wang R., Tam A., Ma X.-J., Erlander M., Sgroi D.C., Rocco J.W., Lingen M.W., Cohen E.E.W., Louis D.N., Settleman J., Haber D.A.
A genome-wide screen for microdeletions reveals disruption of polarity complex genes in diverse human cancers.
Cancer Res. 70:2158-2164(2010)
PubMed=22460905; DOI=10.1038/nature11003
Barretina J.G., Caponigro G., Stransky N., Venkatesan K., Margolin A.A., Kim S., Wilson C.J., Lehar J., Kryukov G.V., Sonkin D., Reddy A., Liu M., Murray L., Berger M.F., Monahan J.E., Morais P., Meltzer J., Korejwa A., Jane-Valbuena J., Mapa F.A., Thibault J., Bric-Furlong E., Raman P., Shipway A., Engels I.H., Cheng J., Yu G.-Y.K., Yu J.-J., Aspesi P. Jr., de Silva M., Jagtap K., Jones M.D., Wang L., Hatton C., Palescandolo E., Gupta S., Mahan S., Sougnez C., Onofrio R.C., Liefeld T., MacConaill L.E., Winckler W., Reich M., Li N.-X., Mesirov J.P., Gabriel S.B., Getz G., Ardlie K., Chan V., Myer V.E., Weber B.L., Porter J., Warmuth M., Finan P., Harris J.L., Meyerson M.L., Golub T.R., Morrissey M.P., Sellers W.R., Schlegel R., Garraway L.A.
The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity.
Nature 483:603-607(2012)
PubMed=22949125; DOI=10.1002/ijc.27822
Pawlowski R., Muhl S.M., Sulser T., Krek W., Moch H., Schraml P.
Loss of PBRM1 expression is associated with renal cell carcinoma progression.
Int. J. Cancer 132:E11-E17(2013)
PubMed=25485619; DOI=10.1038/nbt.3080
Klijn C., Durinck S., Stawiski E.W., Haverty P.M., Jiang Z.-S., Liu H.-B., Degenhardt J., Mayba O., Gnad F., Liu J.-F., Pau G., Reeder J., Cao Y., Mukhyala K., Selvaraj S.K., Yu M.-M., Zynda G.J., Brauer M.J., Wu T.D., Gentleman R.C., Manning G., Yauch R.L., Bourgon R., Stokoe D., Modrusan Z., Neve R.M., de Sauvage F.J., Settleman J., Seshagiri S., Zhang Z.-M.
A comprehensive transcriptional portrait of human cancer cell lines.
Nat. Biotechnol. 33:306-312(2015)
PubMed=25877200; DOI=10.1038/nature14397
Yu M., Selvaraj S.K., Liang-Chu M.M.Y., Aghajani S., Busse M., Yuan J., Lee G., Peale F.V., Klijn C., Bourgon R., Kaminker J.S., Neve R.M.
A resource for cell line authentication, annotation and quality control.
Nature 520:307-311(2015)
PubMed=26589293; DOI=10.1186/s13073-015-0240-5
Scholtalbers J., Boegel S., Bukur T., Byl M., Goerges S., Sorn P., Loewer M., Sahin U., Castle J.C.
TCLP: an online cancer cell line catalogue integrating HLA type, predicted neo-epitopes, virus and gene expression.
Genome Med. 7:118.1-118.7(2015)
PubMed=26972028; DOI=10.1016/j.jprot.2016.03.008
Masuishi Y., Kimura Y., Arakawa N., Hirano H.
Identification of glycosylphosphatidylinositol-anchored proteins and omega-sites using TiO2-based affinity purification followed by hydrogen fluoride treatment.
J. Proteomics 139:77-83(2016)
PubMed=27141528; DOI=10.1016/j.dib.2016.04.001
Masuishi Y., Kimura Y., Arakawa N., Hirano H.
Data for identification of GPI-anchored peptides and omega-sites in cancer cell lines.
Data Brief 7:1302-1305(2016)
PubMed=27993170; DOI=10.1186/s12943-016-0565-8
Brodaczewska K.K., Szczylik C., Fiedorowicz M., Porta C., Czarnecka A.M.
Choosing the right cell line for renal cell cancer research.
Mol. Cancer 15:83.1-83.15(2016)
PubMed=28196595; DOI=10.1016/j.ccell.2017.01.005
Li J., Zhao W., Akbani R., Liu W.-B., Ju Z.-L., Ling S.-Y., Vellano C.P., Roebuck P., Yu Q.-H., Eterovic A.K., Byers L.A., Davies M.A., Deng W.-L., Gopal Y.N.V., Chen G., von Euw E.M., Slamon D.J., Conklin D., Heymach J.V., Gazdar A.F., Minna J.D., Myers J.N., Lu Y.-L., Mills G.B., Liang H.
Characterization of human cancer cell lines by reverse-phase protein arrays.
Cancer Cell 31:225-239(2017)
PubMed=28489074; DOI=10.1038/ncomms15165
Sinha R., Winer A.G., Chevinsky M., Jakubowski C., Chen Y.-B., Dong Y.-Y., Tickoo S.K., Reuter V.E., Russo P., Coleman J.A., Sander C., Hsieh J.J.-D., Hakimi A.A.
Analysis of renal cancer cell lines from two major resources enables genomics-guided cell line selection.
Nat. Commun. 8:15165.1-15165.10(2017)
PubMed=30894373; DOI=10.1158/0008-5472.CAN-18-2747
Dutil J., Chen Z.-H., Monteiro A.N.A., Teer J.K., Eschrich S.A.
An interactive resource to probe genetic diversity and estimated ancestry in cancer cell lines.
Cancer Res. 79:1263-1273(2019)
PubMed=31068700; DOI=10.1038/s41586-019-1186-3
Ghandi M., Huang F.W., Jane-Valbuena J., Kryukov G.V., Lo C.C., McDonald E.R. III, Barretina J.G., Gelfand E.T., Bielski C.M., Li H.-X., Hu K., Andreev-Drakhlin A.Y., Kim J., Hess J.M., Haas B.J., Aguet F., Weir B.A., Rothberg M.V., Paolella B.R., Lawrence M.S., Akbani R., Lu Y.-L., Tiv H.L., Gokhale P.C., de Weck A., Mansour A.A., Oh C., Shih J., Hadi K., Rosen Y., Bistline J., Venkatesan K., Reddy A., Sonkin D., Liu M., Lehar J., Korn J.M., Porter D.A., Jones M.D., Golji J., Caponigro G., Taylor J.E., Dunning C.M., Creech A.L., Warren A.C., McFarland J.M., Zamanighomi M., Kauffmann A., Stransky N., Imielinski M., Maruvka Y.E., Cherniack A.D., Tsherniak A., Vazquez F., Jaffe J.D., Lane A.A., Weinstock D.M., Johannessen C.M., Morrissey M.P., Stegmeier F., Schlegel R., Hahn W.C., Getz G., Mills G.B., Boehm J.S., Golub T.R., Garraway L.A., Sellers W.R.
Next-generation characterization of the Cancer Cell Line Encyclopedia.
Nature 569:503-508(2019)
PubMed=31978347; DOI=10.1016/j.cell.2019.12.023
Nusinow D.P., Szpyt J., Ghandi M., Rose C.M., McDonald E.R. III, Kalocsay M., Jane-Valbuena J., Gelfand E.T., Schweppe D.K., Jedrychowski M.P., Golji J., Porter D.A., Rejtar T., Wang Y.K., Kryukov G.V., Stegmeier F., Erickson B.K., Garraway L.A., Sellers W.R., Gygi S.P.
Quantitative proteomics of the Cancer Cell Line Encyclopedia.
Cell 180:387-402.e16(2020)
一、目的与意义 学习使用限制性内切酶切割带有相应酶切位点的PCR产物,并将克隆载体与外源DNA片段进行体外连接,以构建重组DNA。 二、实验原理 外源基因(DNA片段)很难直接通过细胞膜进入受体细胞,即使进入,也会受到细胞内限制性酶的作用而分解。要将外源DNA片段导入受体细胞,必须选择适当的载体(Vector)。 载体是携带外源基因进入受体细胞的工具,作为载体的DNA分子,需具备三个基本
科:临床医学和基础医学之间的桥梁--九江市第一人民医院科专家访谈九江市第一人民医院科是该院重点建设医技科室,同时也承担九江市临床中心职能,依托九江医学专业委员会,担负全市临床实室的指导,培训,质控,考评,收等项工作。现有专业技术人员42人,其中副主任技师2人,中级职称21人,硕士研究生2人,大学本科28人。记者:柏教授您好,科对患者们来说算得上是既熟悉又陌生了,如果一提这个科室肯定对于绝大多数人会直观的说是“化的方”,那不知道这样说是否片面或者说就是错误的认知,您能不能给读者们客观的介绍一下
。36人中有25人伴有胸骨后烧灼感,服药一周后均全部缓解。溃疡愈合情况:经1个疗程的治疗, 胃溃疡 病人有7人获愈,1人好转。十二指肠溃疡者有11人获愈,2人好转。溃疡愈合率为75%,总有效率为91.7%。胃炎好转情况:服药一个疗程后,12例并发胃炎有9例获愈,3例好转。浅表性胃炎有6人获愈,2人好转。萎缩性胃炎有2人获愈,1人好转。胃炎治愈率70.8%,总有效率为95.8%。 3 讨论 得乐冲剂主要含枸橼酸铋钾,又称胶态
 技术资料
技术资料暂无技术资料 索取技术资料