相关产品推荐更多 >
万千商家帮你免费找货
0 人在求购买到急需产品
- 详细信息
- 文献和实验
- 技术资料
- 库存:
100
- 国食药监械注册号:
无
- 保修期:
12个月
- 现货状态:
3天
- 供应商:
玉研科学仪器公司
玉研仪器公司作为Mouse Ox Plus 小动物脉搏血氧仪产品的中国区总代理,该仪器适用于小鼠、大鼠、豚鼠、兔子等动物,可以在清醒或麻醉状态下测量动物的脉搏、血氧、呼吸、体温等多种生理参数。
· 小动物脉搏血氧仪可用于多参数数据的采集或手术监护,可拓展多通道大鼠、小鼠的生理信号测量仪;
· 以无创的方式测量小动物(幼鼠,小鼠,大鼠,豚鼠,兔, 等)的血氧饱和度、脉搏频率、呼吸频率、脉搏幅度、呼吸幅度和体温;
· 除体温外,所有测量都是通过一个无创的感应器。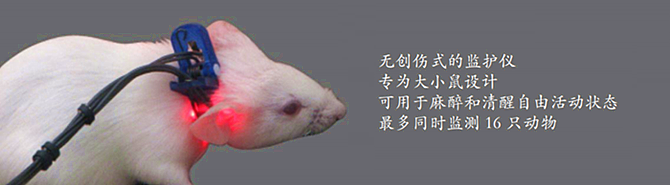
所有测量都是通过一个无创的感应器完成,准确、方便、高效。
型号:MouseOx 正在对麻醉状态下的小鼠进行测试
有多种探头可供选择:
· 根据实验需求:可选择大鼠型探头、小鼠型探头;
· 根据动物状态:可选择清醒活动状态连续测量和麻醉(或手术)状态测量探头;
· 根据动物数量:有多通道适配器可供选择,同时检测多只动物的生命体征;
· 根据使用环境:可选择核磁环境适用的无磁探头;
主要功能:
· 小动物手术术中监测(保证适当的麻醉深度,防止手术中缺氧)
· 一个无创传感器获得多个生命信号 (动脉血氧饱和度,心率,呼吸频率,脉搏幅度,呼吸幅度)
· 心肺功能参数记录
· 输出模拟数据
经过验证的准确度:
使用有创血气采样测量结果与无创 MouseOx 测量结果的比较, 对比表明, 两者具有很好的线性关系。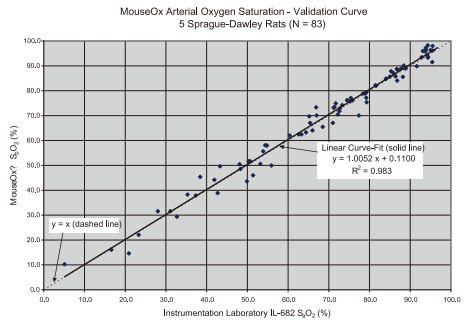
脉搏、血氧、呼吸等心肺监测参数:
· 脉波频率在90到900BPM范围内监测 (每分钟心跳, Beat per minutes, BPM)
· 血氧饱和度监测范围:0% 到100% 动脉血氧饱和度;
· 血氧饱和度监测误差:<1.5% 横跨整个监测范围;
· 血氧饱和度监测反应时间:实时报告动脉血氧饱和度, 在每次心跳以后0.72秒屏幕刷新;
· 呼吸频率监测范围:每分钟 25到450 次;
· 监测反应时间:呼吸率每1.7秒向用户报告, 移动报告的值是10次呼吸的的平均数;
· 无创伤监测脉搏充盈度以估量血流量的变化;
· 脉搏监测范围:内径0到800微米的徽小血管;
· 监测误差:< 2.4%横跨整个监测范围;
· 监测反应时间:脉搏充盈度实时向用户报告, 在每次心跳以后,0.72秒屏幕刷新,刷新屏幕显示被测量的所有脉搏充盈度;
· 无创伤监测动物呼吸幅度的变化;
· 呼吸幅度监测范围:每分钟25到450次;
· 呼吸幅度监测反应时间:呼吸率每1.7秒向用户报告, 移动报告的值是10次呼吸的的平均数;
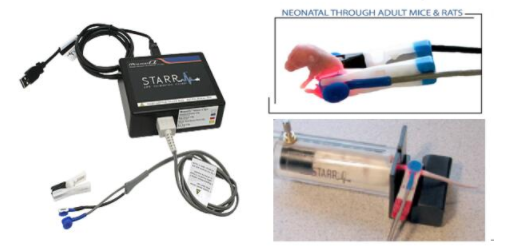
多钟测试探头可选:
根据需要,可选择老鼠清醒状态下使用的颈部探头,麻醉状态下使用的足部探头和大腿探头
以下是使用有创血气采样测量结果与无创MouseOx测量结果的比较
可实现大鼠、小鼠清醒活动状态下进行测量
软件界面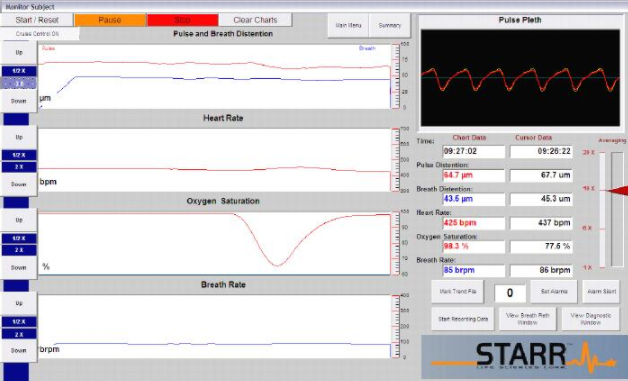
小动物脉搏血氧监护仪的部分参考文献:
1. Albéri, L., Lintas, A., Kretz, R., Schwaller, B., & Villa, A. E. (2013). The calcium-binding protein parvalbumin modulates the firing 1 properties of the reticular thalamic nucleus bursting neurons. Journal of neurophysiology, 109(11), 2827-2841.
2. Sonati, T., Reimann, R. R., Falsig, J., Baral, P. K., O’Connor, T., Hornemann, S., Aguzzi, A. (2013). The toxicity of antiprion antibodies is mediated by the flexible tail of the prion protein. Nature, 501(7465), 102-106.
3. Ali, I., O’Brien, P., Kumar, G., Zheng, T., Jones, N. C., Pinault, D., O’Brien, T. J. (2013). Enduring Effects of Early Life Stress on Firing Patterns of Hippocampal and Thalamocortical Neurons in Rats: Implications for Limbic Epilepsy. PLOS ONE, 8(6), e66962.
4. Bell, L. A., Bell, K. A., & McQuiston, A. R. (2013). Synaptic Muscarinic Response Types in Hippocampal CA1 Interneurons Depend on Different Levels of Presynaptic Activity and Different Muscarinic Receptor Subtypes. Neuropharmacology.
5. Bolzoni, F., Bączyk, M., & Jankowska, E. (2013). Subcortical effects of transcranial direct current stimulation (tDCS) in the rat. The Journal of Physiology.
6. Bolzoni, F., Bączyk, M., & Jankowska, E. (2013). Subcortical effects of transcranial direct current stimulation (tDCS) in the rat. The Journal of Physiology.
7. Babaei, P., Tehrani, B. S., & Alizadeh, A. (2013). Effect of BDNF and adipose derived stem cells transplantation on cognitive deficit in Alzheimer model of rats. Journal of Behavioral and Brain Science, 3, 156-161.
8. Gilmartin, M. R., Miyawaki, H., Helmstetter, F. J., & Diba, K. (2013). Prefrontal Activity Links Nonoverlapping Events in Memory. The Journal of Neuroscience, 33(26), 10910-10914.
9. Feng, L., Sametsky, E. A., Gusev, A. G., & Uteshev, V. V. (2012). Responsiveness to nicotine of neurons of the caudal nucleus of the solitary tract correlates with the neuronal projection target. Journal of Neurophysiology, 108(7), 1884-1894.
10. Clarner, T., Diederichs, F., Berger, K., Denecke, B., Gan, L., Van der Valk, P., Kipp, M. (2012). Myelin debris regulates inflammatory responses in an experimental demyelination animal model and multiple sclerosis lesions. Glia, 60(10), 1468-1480.
11. Girardet, C., Bonnet, M. S., Jdir, R., Sadoud, M., Thirion, S., Tardivel, C., Troadec, J. D. (2011). Central inflammation and sickness-like behavior induced by the food contaminant deoxynivalenol: A PGE2-independent mechanism.Toxicological Sciences, 124(1), 179-191.
12. Hruška-Plocháň, M., Juhas, S., Juhasova, J., Galik, J., Miyanohara, A., Marsala, M., Motlik, J. (2010). A27 Expression of the human mutant huntingtin in minipig striatum induced formation of EM48+ inclusions in the neuronal nuclei, cytoplasm and processes. Journal of Neurology, Neurosurgery & Psychiatry, 81(Suppl 1), A9-A9.
13. Brooks, S., Jones, L., & Dunnett, S. B. (2010). A29 Frontostriatal pathology in the (C57BL/6J) YAC128 mouse uncovered by the operant delayed alternation task. Journal of Neurology, Neurosurgery & Psychiatry, 81(Suppl 1), A9-A10.
14. Yu, L., Metzger, S., Clemens, L. E., Ehrismann, J., Ott, T., Gu, X., Nguyen, H. P. (2010). A28 Accumulation and aggregation of human mutant huntingtin and neuron atrophy in BAC-HD transgenic rat. Journal of Neurology, Neurosurgery & Psychiatry, 81(Suppl 1), A9-A9.
15. Baxa, M., Juhas, S., Pavlok, A., Vodicka, P., Juhasova, J., Hruška-Plocháň, M., Motlik, J. (2010). A26 Transgenic miniature pig as an animal model for Huntington’s disease. Journal of Neurology, Neurosurgery & Psychiatry, 81(Suppl 1), A8-A9.
敬请关注玉研仪器微信号:
风险提示:丁香通仅作为第三方平台,为商家信息发布提供平台空间。用户咨询产品时请注意保护个人信息及财产安全,合理判断,谨慎选购商品,商家和用户对交易行为负责。对于医疗器械类产品,请先查证核实企业经营资质和医疗器械产品注册证情况。
 文献和实验
文献和实验1.Suzuki R, Yamasoba D, Kimura I, et al. Attenuated fusogenicity and pathogenicity of SARS-CoV-2 Omicron variant[J]. Nature, 2022, 603(7902): 700-705.
2.Wang G, Wen B, Deng Z, et al. Endothelial progenitor cells stimulate neonatal lung angiogenesis through FOXF1-mediated activation of BMP9/ACVRL1 signaling[J]. Nature communications, 2022, 13(1): 1-16.
3.Aiba I, Noebels J L. Kcnq2/Kv7. 2 controls the threshold and bi-hemispheric symmetry of cortical spreading depolarization[J]. Brain, 2021, 144(9): 2863-2878.
4.Bachus H, Kaur K, Papillion A M, et al. Impaired tumor-necrosis-factor-α-driven dendritic cell activation limits lipopolysaccharide-induced protection from allergic inflammation in infants[J]. Immunity, 2019, 50(1): 225-240. e4.
5.Han W, Tellez L A, Perkins M H, et al. A neural circuit for gut-induced reward[J]. Cell, 2018, 175(3): 665-678. e23.
6.Lai A Y, Dorr A, Thomason L A M, et al. Venular degeneration leads to vascular dysfunction in a transgenic model of Alzheimer’s disease[J]. Brain, 2015, 138(4): 1046-1058.
7.Thomas G M, Carbo C, Curtis B R, et al. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice[J]. Blood, The Journal of the American Society of Hematology, 2012, 119(26): 6335-6343.
8.Dorr A, Sahota B, Chinta L V, et al. Amyloid-β-dependent compromise of microvascular structure and function in a model of Alzheimer’s disease[J]. Brain, 2012, 135(10): 3039-3050.
9.Abt M C, Osborne L C, Monticelli L A, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity[J]. Immunity, 2012, 37(1): 158-170.
Fung Y L, Kim M, Tabuchi A, et al. Recipient T lymphocytes modulate the severity of antibody-mediated transfusion-related acute lung injury[J]. Blood, The Journal of the American Society of Hematology, 2010, 116(16): 3073-3079.
【公告】丁香通试用中心:10月免费试用装精选(10月18日有更新)
200ul低吸附盒装吸头 进口TS3000转染试剂 纳米微粒 非脂质体 其他推荐: 单组分TMB显色液 Peroxidase Conjugated Goat Anti-Rabbit IgG (Min Hu, Ms) 二抗 96通道个人型半自动移液系统 小动物气体麻醉机,大小鼠气体吸入式麻醉机 四通道实时荧光定量PCR检测系统 Tanon-5200化学发光成像系统(包邮) 优质微晶纤维素MCC(进口) 罗恩1-10ml电动移液器 EpiScript逆转录酶 western
自动原位杂交仪 吸入式小动物麻醉机 细胞计数仪 超微量分光光度计
大小鼠转棒仪该仪器用于研究药物对动作协调性和抗疲劳特性的影响,对相关药物筛选有重要价值。实验时将动物放置在滚筒上并避免滑落,转动滚筒后,如果动物滑落下来就会相应停止下面的传感平台进行结果记录,可以同时进行五个大鼠或小鼠实验,大鼠采用直径3.75英寸的滚筒,小鼠采用直径为1.25英寸的滚筒。仪器采用数字控制: 5个标准通道,测试时间可调; 启动速度可调,最终速度可调; 加速度可调,前进反转两种模式选择; 测量距离可记录,标准计算机打印口输出; RS232 串口输出。大小鼠疲劳测试
 技术资料
技术资料