相关产品推荐更多 >
万千商家帮你免费找货
0 人在求购买到急需产品
- 详细信息
- 文献和实验
- 技术资料
- 库存:
详询
- 供应商:
北京百奥创新科技有限公司
- 英文名:
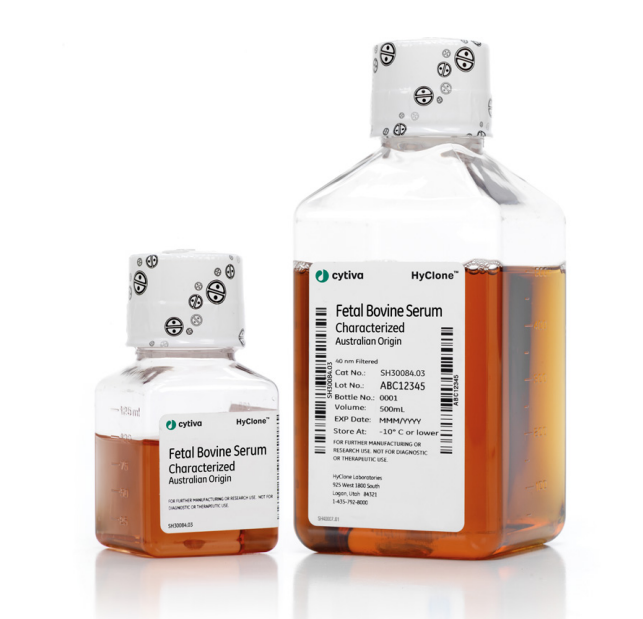
HyClone Characterized FBS, AUS Origin, Processed in NZ, 100 mL, Irradiated 25-40kGy
- 规格:
具体价格请联系我们
产品货号:SH30084.02IR25-40;
处理方式:3×100 nm滤膜过滤;
地区来源:澳大利亚;
物种来源:;
内毒素水平:内毒素水平:≤ 25 EU/mL;
血红素水平:< 25 mg/dL
热灭活:未灭活;
射线处理:处理;
保存条件:≤-10℃;
运输方式:干冰运输;
详细介绍:This premium HyCloneTM Fetal Bovine Australian-origin Serum is well-characterized, triple 100 nm filtered, virus-tested to 9 CFR standard, and traceability-certified (ISIA, Oritain). Australian origin: benefit from Australian husbandry methods and certified traceability. Characterized: assayed for a variety of critical components and biochemical characteristics. Endotoxin-tested: certificate of analysis confirms ≤ 25 EU/mL endotoxin. Triple-filtered: serial 100 nm pore size-rated filtering for reliable microbial depletion. Post-filtration treatments: γ-irradiated and heat-inactivated FBS options also available. Virus-tested: broad virus panel testing in accordance with 9 CFR 113.53. Fetal bovine serum (FBS) is a nutrient-rich cell culture supplement that is high in growth factors and low in antibodies, compared to non-fetal sera. Why choose HyCloneTM Australian Origin FBS? HyClone Australian Origin serum is sourced from Australian abattoirs approved by the U.S. Department of Agriculture (USDA) or Australian Quarantine and Inspection Service (AQIS). The Australian methods of animal husbandry exhibit excellent animal nutrition and healthcare. Australia is an isolated continent, thus making animal disease and control management easier than in most areas of the world. Characterized FBS for cell culture HyClone Characterized FBS is an excellent alternative to more expensive defined sera, and meets the requirements of most discriminating FBS users. The final serum is assayed for important proteins, hormones, growth factors, metabolites, and nutrients that support healthy cell cultures. Assay results are included in the certificate of analysis and biochemical assay list. Additional testing, including EMEA-conforming testing, is also available. Have Confidence in Security of Supply with Secondary Sourcing Fetal Bovine Serum from a single origin may be limited, so it’s important to qualify FBS products from other countries to increase your security of supply. HyClone Characterized FBS from United States (US), New Zealand (NZ) and Australia (AUS) have been evaluated and tested to show equivalent performance. Read our full study. Bacterial endotoxin-tested Endotoxin contamination is a significant concern in research and manufacturing, especially when producing cell culture-derived products for use in humans or animals. The presence of endotoxin in cell cultures can also have adverse and unpredictable effects on cell growth, function and phenotype that can compromise research results. Our patented closed-system blood collection method and rigorous quality control testing ensure that HyClone Characterized FBS meets industry standards of ≤ 25 EU/mL endotoxin. The final product also contains ≤ 25 mg/dL hemoglobin, which is an indicator of appropriate handling during collection and transport. Virus-tested and quality-assured for reduced contamination risk HyClone Characterized FBS is triple-filtered through serial 100 nm (0.10 μm) pore size-rated filters, which has become an industry standard for cell culture grade FBS. Before dispensing, each lot of serum is pooled using true pool technology to minimize variability from bottle to bottle. The final serum is virus-tested according to 9 CFR 113.53 to demonstrate that there are no detectable levels of specified viruses, pathogenic agents, and hemadsorbing agents. Robust sterility testing further confirms that the final product contains no detectable growth of bacteria or fungi (current USP) and no detectable mycoplasma. Post-filtration treatments for added process security Gamma-irradiated and heat-inactivated FBS options are available to further reduce the risk of adventitious agents, particularly when manufacturing products for use in humans or animals. The irradiation process is validated to deliver a minimum dose of 25 kGy. Regulatory and supply chain security For decades, Cytiva's HyClone™ premium bovine serum products have exceeded industry standards for quality, purity, and regulatory compliance. HyClone™ serum processes are traceability traceability-certified by the International Serum Industry Association (ISIA) and, . Iin addition, Cytiva has chosen to be an early adopter of scientific traceability by Oritain to prove the country of origin of their premium bovine serum products from the United States, Australia and New Zealand. Premium bovine serum samples from different regions have been analyzed and Oritain has created a database of the unique ‘Origin Fingerprint’ that is exclusive to each country of origin.
风险提示:丁香通仅作为第三方平台,为商家信息发布提供平台空间。用户咨询产品时请注意保护个人信息及财产安全,合理判断,谨慎选购商品,商家和用户对交易行为负责。对于医疗器械类产品,请先查证核实企业经营资质和医疗器械产品注册证情况。
 文献和实验
文献和实验-044,是100nm过滤3次,内毒素和血红蛋白含量都可以与Hyclone的SH30070.03相媲美。也可以用于培养大部分的干细胞 系。同样是美国来源,所以渠道特殊。 3.TCB的特级胎牛血清 TCB(Tissue Culture Biologicals)公司作为一家生物行业类的著名供应商,成立于1978年,大部分血清的采集和加工都在加利福尼亚。每一批的原材料都经过严格筛选,所有的产品都在FDA认证的cGMP车间进行! TCB 公司一贯重视产品质量,为客户提供
Gemini 胎牛血清,透析型胎牛血清,炭吸附胎牛血清,辐照型胎牛血清。 Gemini 公司,成立于 1985 年,位于加利福尼亚南部 Calabasas 市,1999 年,迁至加利福尼亚北部 Woodland 市。 Gemini 始终以「为客户提供高质量产品」为首要目标,尤其在细胞培养用胎牛血清方面,Gemini 产品质量好、价格低,和同类产品相比竞争力强。 Gemini 公司在全球销售的动物血清产品,全部来自于澳洲的子公司,血源主要为澳大利亚、新西兰、巴拿马、乌拉圭、巴西
的质量不如GIBCO(同类血清)。国产的杭州四季青和甘肃民海(中美和资)不错。有些细胞(如:sf9,293还有其他一些元代细胞),用Gibco的比其他两种好很多,会大大加速试验进度。对于其他一些细胞(如:vero,MDCK,pk)四季青,Hyclone的小牛血清足矣!另外,Gibco的还要看产地。 2、问:最近要订一瓶胎牛血清,实验室之前都在用小牛血清,没有买过胎牛血清。请问哪个公司的好一些?问过试剂公司,有两种:一种是PAA的(分普通级和优级),一种是Hyclone的(只有普通级,而且是新西兰产
 技术资料
技术资料







