相关产品推荐更多 >
万千商家帮你免费找货
0 人在求购买到急需产品
- 详细信息
- 文献和实验
- 技术资料
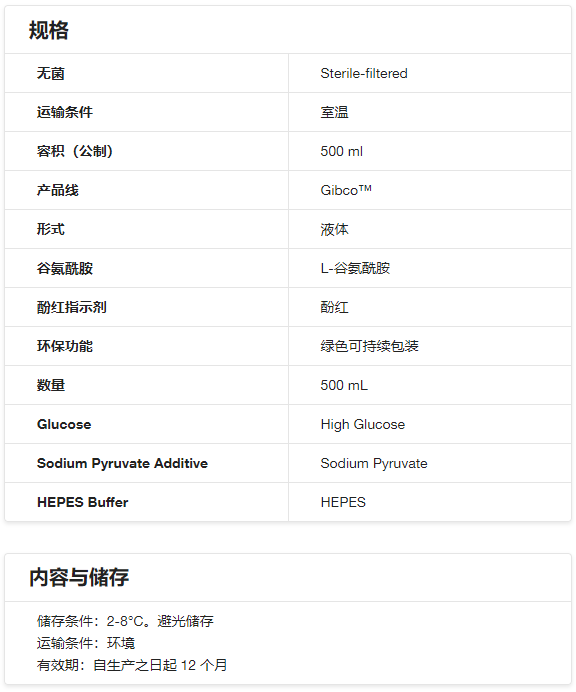
- 规格:
500ml
Iscove's Modified Dulbecco's Medium (IMDM) is well suited for rapidly proliferating, high-density cell cultures, including Jurkat, COS-7, and macrophage cells. Life Technologies offers a variety of Gibco® IMDM modifications for a range of cell culture applications. Find the right formulation using the media selector tool.
This IMDM is modified as follows:
| With | Without |
| • L-glutamine | • α-thioglycerol |
| • Phenol Red | • 2-mercaptoethanol |
The complete formulation is available.
IMDM, a modification of Dulbecco's Modified Eagle Medium, includes selenium as well as additional amino acids and vitamins. In addition, this unique medium lacks iron, with potassium nitrate replacing ferric nitrate.
Product Intended Use
For in vitro diagnostic use. CAUTION: Not for human or animal therapeutic use. Uses other than the intended use may be a violation of local law.
cGMP Manufacturing and Quality System
Gibco® IMDM is manufactured at a cGMP compliant facility, located in Grand Island, New York. The facility is registered with the FDA as a medical device manufacturer and is certified to ISO 13485 and ISO 9001 standards. For supply chain continuity, Life Technologies offers a comparable Gibco® IMDM product made in our Scotland facility (21980-065). This facility is registered with the FDA as a medical device manufacturer and is certified to the ISO 13485 standard.
IMDM contains no proteins, lipids, or growth factors. Therefore, IMDM requires supplementation, commonly with 10% Fetal Bovine Serum (FBS). IMDM uses a sodium bicarbonate buffer system (3.024 g/L) and therefore requires a 5-10% CO2 environment to maintain physiological pH.
在中国境内, 本产品仅限于非临床科研用途, 产品信息以中文为准。 (In China territory, the product is for Research Use only in non-clinical unit, product information in Chinese shall prevail.)
风险提示:丁香通仅作为第三方平台,为商家信息发布提供平台空间。用户咨询产品时请注意保护个人信息及财产安全,合理判断,谨慎选购商品,商家和用户对交易行为负责。对于医疗器械类产品,请先查证核实企业经营资质和医疗器械产品注册证情况。
 文献和实验
文献和实验Procedure for Transfection of Mammalian Cells
Materials: Lipofectamine IMDM containing 10% fetal bovine serum, 1% glutamine, 1% aa IMDM containing 1% glutamine
实验大纲 骨髓间充质干细胞是多能干细胞。因此,作为再生医学的细胞来源,它是活性研究和临床应用的目标。当骨髓液或骨髓细胞接种在培养皿上时,间充质干细胞会作为粘附细胞增殖。因此,间充质干细胞可以从漂浮的血细胞中分离出来。在此,我们报告了使用 Evident Prov CM20 培养监测系统对间充质干细胞原代培养的远程监测结果。 试验程序 使用补充了 10% 胎牛血清(FBS)和 1% 青霉素链霉素的 Iscove 改良 Dulbecco 培养基(IMDM),将市售人骨髓细胞以 950,000
Procedure for Transfection of Mammalian Cells Materials: Lipofectamine IMDM containing 10% fetal bovine serum, 1% glutamine, 1% aa IMDM containing 1% glutamine IMDM containing 20% fetal bovine serum, 1% glutamine, 1% aa
 技术资料
技术资料暂无技术资料 索取技术资料