北京云肽生物科技有限公司
5 年
手机商铺
- NaN
- 0.3999999999999999
- 1.4
- 0.3999999999999999
- 3.4
- 查看全部分类
- 天津灏洋/TBD
- 康宁 corning
- 伯乐Bio-Rad
- 爱思进 Axygen
- 艾本德Eppendorf
- 密理博Millipore
- falcon
- 徕卡(Leica)
- Cytiva 思拓凡
- 赛默飞 Thermo
- Sigma
- BD Difco
- BD
- Omega
- Sigma
- LIFE
- 天根
- ScienCell
- 赛洛捷克
- 经销品牌
- OMEGA
- Cygnus
- NobleRyder
- 经销品牌2
- 经销品牌3
- 经销品牌3
- DLAB
- 经销品牌2
- 经销品牌4
- 经销品牌5
- Epredia
- 经销品牌7
- 硕华
- Gilson/吉尔森
- 大龙/DLAB
- Sigma/西格玛
- Merck/默克
- sysmex
- SERVA
- HIMEDIA
- Calbiochem
- Biovision
- 三菱
- 美国Nasco
- 麦迪康 Medicom
- 施睿康SRITRANG
- 赛多利斯
- Asone
- 格拉文Gloveon
- 法国Interscience
- Whirl-Pak
- Wheaton
- Venabio
- Vitlab
- Anios
- 3M
- Bionova
- CRYOBANK
- Corning
- Biolog
- Crosstex
- Bel-Art
- Biologix
- Chromagar/科玛嘉
- VP Stericlin
- Bio-kont
- Biomerieux/梅里埃
- Chemdye
- Interscience
- IDEXX 爱德士
- DWS Life Science
- DWK Life Science
- Don Whitley Scientific
- Greiner
- MesaLabs
- Merck
- Microbank
- Millipore
- Terragene
- SteriClin
- Shield Scientific
- Texwipe
- Pro-Loops
- Prolex
- ProSpecT
- phygen®
- Remel BactiDrop
- Remel BactiCard
- Shunyoubio
- Simax
- SteriTec
- Seroat
- Nasco
- Sterileware
- Remel
- Sartorius
- G-Biosciences
- 源井生物
- 华雅思创
技术资料/正文
BEBP02-054Q LONZA X-VIVO 15 免疫细胞无血清培养基
16 人阅读发布时间:2026-01-03 08:10
BEBP02-054Q LONZA X-VIVO 15 免疫细胞无血清培养基
北京云肽生物科技代理的LONZA X-VIVO 15 免疫细胞培养基(货号:BEBP02-054Q),作为LONZA最畅销的无血清培养基,可为免疫细胞提供营养平衡完善的体外培养环境,已成功应用于T细胞、造血干细胞、单核细胞、巨噬细胞、CIK、自然杀伤细胞、外周血淋巴细胞、树突状细胞、淋巴因子激活杀伤细胞、肿瘤浸润淋巴细胞、粒细胞的培养中。
LONZA X-VIVO 15 培养基,不含外源生长因子、人工细胞增殖刺激因子、蛋白激酶C刺激剂,所有成分明确,含有重组人胰岛素、重组人转铁蛋白和白蛋白,并针对肿瘤浸润淋巴细胞(TIL)的体外无血清培养进行了优化,近年来在众多CAR-T研究中得到使用。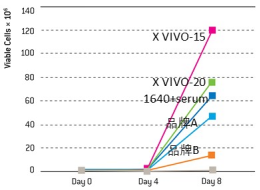
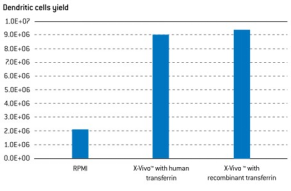
Serum-free media outperforms serum-based media and maintains phenotype, with secondary suppliers for cytokines.
T-cells (1x106) were stimulated with CD3/CD28 Dynabeads® in both 6-well plates (5 ml) and a closed system breathable vessel
(35 ml) with different media. The serum-free X-VIVO media supported the largest expansion of T-cells, comparable to RPMI
supplemented with human serum in both open and closed systems and outperforming several serum-free media alternatives
LONZA X-VIVO 15 已在美国FDA进行备案。 生产用X-VIVO 15培养基由拥有质量管理认证的欧洲或美国工厂生产,严格按照cGMP标准,不含抗生素、不含酚红,且全部标记为FFM (For Further Manufacture)。用于科研及研发用途的X-VIVO 15培养基,添加抗生素及酚红,并标记为RUO(Research Use Only)。
|
产品名称 |
订货号 |
规格 |
级别 |
备注 |
|
LONZA X-VIVO 15 培养基,含L-谷氨酰胺,不含酚红,不含庆大霉素 |
BEBP02-054Q |
1L |
GMP |
原04-744Q已停产 |
|
LONZA X-VIVO 15 培养基,含L-谷氨酰胺,含酚红,含庆大霉素 |
04-418Q |
1L |
|
|
瑞士LONZA集团,在全球拥有100余个生产基地和办事处,全球员工14500余人,是著名的生物技术、制药、特殊成分供应商,多年来为基因治疗研究、过继免疫治疗研究及其他细胞疗法基础研究开发了众多研究工具。
LONZA X-VIVO 15 相关文献
1. Highly efficient therapeutic gene editing of human hematopoietic stem cells.(NatureMedicine,2019)
2. Bacteria-free minicircle DNA system to generate integration-free CAR-T cells.(J Med Genetics,2019,56: 10-17)
3. Genome-wide CRISPR Screens in Primary Human T Cells Reveal Key Regulators of Immune Function.(Cell,2018,175(7):1985-1971)
4. A high-fidelity Cas9 mutant delivered as a ribonucleoprotein complex enables efficient gene editing in human hematopoietic stem and progenitor cells.(Nature Medicine,2018,24(8): 1216-1224)
5. Clinical grade manufacturing of genetically modified, CAR-expressing NK-92 cells for the treatment of ErbB2-positive malignancies. (Cancer Immunol Immunother,2018,67(1):25-38)
6. Improved Expansion and In Vivo Function of Patient T Cells by a Serum-free Medium.(Molecular Therapy,2018,8:65-74)
7. Genetic Inactivation of CD33 in Hematopoietic Stem Cells to Enable CAR T Cell Immunotherapy for Acute Myeloid Leukemia.(Cell,2018,173(6):1439-1453)
8. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection.(Nature,2017,543(7643):113-117)
9. A genome-wide CRISPR screen identifies a restricted set of HIV host dependency factors.(Nature Genetics,2016,49(2):193-203)
10. High efficacy and safety of low-dose CD19-directed CAR-T cell therapy in 51 refractory or relapsed B acute lymphoblastic leukemia patients.(Leukemia,2017,31:2587-2593)
11. Co-stimulatory signaling determines tumor antigen sensitivity and persistence of CAR T cells targeting PSCA+ metastatic prostate cancer. (OncoImmunology,2017,7(2))
12. A genome-wide CRISPR screen identifies a restricted set of HIV host dependency factors.(Nature Genetics,2016,49(2):193-203)

