Cayman Chemical Company,Inc.
入驻年限:4 年
- 联系人:
Kristina Whitfield
- 所在地区:
美国
- 业务范围:
试剂、抗体、细胞库 / 细胞培养、ELISA 试剂盒
- 经营模式:
生产厂商
推荐产品
公司新闻/正文
Tuning Ionizable Cationic Lipid Design for Efficacy and Safety
人阅读 发布时间:2023-10-13 12:08
Lipid nanoparticles (LNPs) have the potential to revolutionize nucleic acid-based therapeutics. While many efforts have been made to improve the efficacy of cationic lipids used in LNPs, safety cannot be undermined. Though cationic lipids found early success in delivering nucleic acids, their suitability for therapeutic use is compromised by safety risks. Significant progress in this field has been made possible by designing ionizable cationic lipids with biocompatible and biodegradable functional groups that can be harnessed to improve the safety profile of these lipids without compromising efficacy.
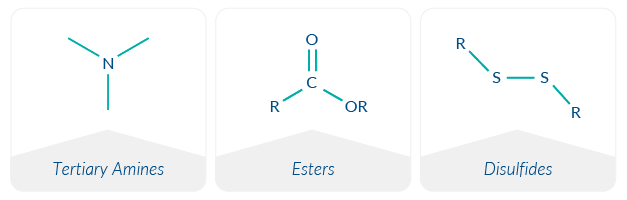
Ionizable head groups for biocompatibility
Early generations of lipids used to deliver nucleic acids included DOTAP and DOTMA.1 These lipids contain a quaternary ammonium head group (Figure 1), which endows the lipid with a permanent cationic charge. Several challenges limit the in vitro and in vivo suitability of these lipids. Cationic particles are rapidly eliminated in systemic circulation, significantly limiting the efficacy of administered therapeutics.2 Furthermore, cationic lipids are incompatible with negatively charged biological membranes, causing cell membrane disruption and resulting in unacceptable cytotoxicity.3,4 Many scientists have experienced this first-hand with cationic transfection reagents in cell-based experiments, having to find a careful balance between target expression (or knockdown) and cytotoxicity.
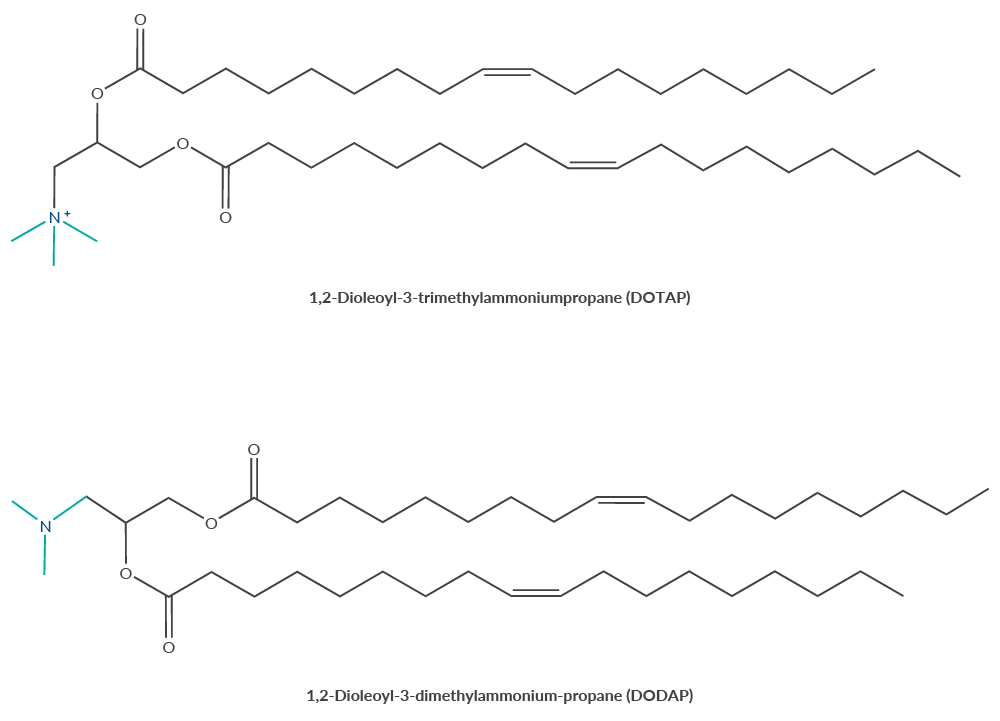
Figure 1. Comparison of quaternary ammonium head groups and ionizable tertiary amine head groups. DOTAP is a cationic lipid containing a quaternary ammonium head group and DODAP is its ionizable derivative.
A major advancement in the delivery of nucleic acid-based therapies was the discovery that ionizable cationic lipids circumvent untoward cytotoxicity. These lipids contain one or more pH-responsive ionizable tertiary amines in the head group, acquiring a cationic charge only when the pH is below the acid-base dissociation constant (pKa).5-8 This transient cationic charge facilitates the encapsulation of negatively charged nucleic acid-based therapeutics in acidic conditions during LNP formulation and its release by the low pH of the endosome. At neutral pH (~7), ionizable cationic lipids have a near-zero charge, improving the stability and avoiding the cytotoxicity observed using LNPs formulated with cationic lipids.9
Ester linkages for biodegradability
Another step forward in the delivery of nucleic acid-based therapeutics was achieved with the incorporation of esters in linker groups or embedded within lipid tails to improve biodegradability.7 Long tissue half-lives have been observed with certain cationic and ionizable cationic lipids, likely contributing to local and systemic adverse effects after administration.10,11 Hence, biodegradable lipids avoid lipid accumulation and untoward cytotoxicity because they are rapidly eliminated.9
Ester and ether linkers were amongst the first linker groups to be studied.1 Ether linker groups were found to be highly potent and stable yet non-biodegradable. Consequently, they are frequently more cytotoxic than their ester-based counterparts. Substitution of ether groups with ester groups improves the biodegradability of the lipid. Esters are stable at physiological pH (such as during LNP distribution in the body) but undergo enzymatic hydrolysis in the cell to hydrophilic products that are rapidly eliminated, limiting tissue accumulation.5,7 Esters are a recurrent structural theme in new ionizable lipids, indicating that biodegradability is a key feature in the design of novel ionizable cationic lipids.
DLin-MC3-DMA, the ester-containing derivative of DLin-DMA, is the ionizable cationic lipid component of patisiran, the first FDA-approved LNP formulation (Figure 2).2 It has been used as a benchmark for the design of novel ester-containing ionizable cationic lipids like SM-102, lipid 5, and L-319 with improved tissue pharmacokinetics and biodegradability.8,10,11
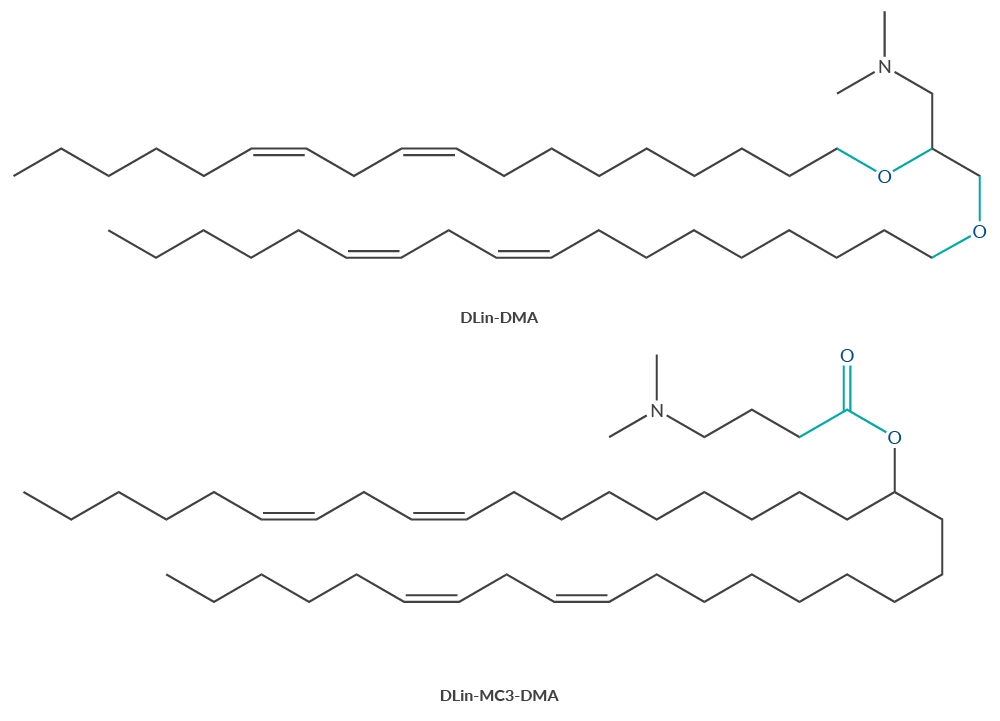
Figure 2. Substitution of the ether groups in DLin-DMA with an ester group produces DLin-MC3-DMA.
DLin-MC3-DMA is the ionizable cationic lipid used in patisiran, the first FDA-approved LNP formulation.
Disulfide bonds for bioreducibility
The incorporation of bioreducible disulfide bonds in the linker and/or hydrophobic tails of novel ionizable cationic lipids is an emerging feature.7 Because reduced glutathione (GSH) is present at high concentrations in the cytosol, the intracellular environment is highly reductive.12 Disulfide bonds within ionizable cationic lipids undergo a disulfide exchange with GSH, resulting in degradation of the disulfide bond and release of the nucleic acid cargo into the cytosol. The accelerated release of nucleic acids conferred by the use of bioreducible lipids boosts the potency of the administered LNP by increasing the efficiency of cargo delivery to the cytosol. In this manner, the use of large amounts of RNA can be avoided, which is both costly and potentially immunogenic.
Cayman's Glutathione Assay Kit or Glutathione Cell-Based Detection Kit (Blue Fluorescence) can be used to measure intracellular GSH levels.
Integrating multiple environmentally responsive features in lipid design
Two classes of ionizable cationic lipids integrate pH-responsive head groups, biodegradable esters, and bioreducible disulfide bonds. SS-cleavable and pH-activated (also known as proton-activated) lipid-like materials (ssPalms) feature a hydrophobic scaffold that can be harnessed to deliver cargo to targeted tissues and subcellular locations.13 These scaffolds include myristic acid (ssPalmM), oleic acid (ssPalmO), vitamin A (ssPalmA), and vitamin E (ssPalmE) and structurally modified derivatives like ssPalmO-Phe.14 A subset of the O-series (where O indicates an ester linker) ionizable cationic lipids also contain bioreducible disulfide bonds. These lipids are named according to the following scheme: R-OXB, where R is the identity of the amine head, X represents the length of the hydrophobic tail, and B stands for the bioreducible disulfide bond (Figure 3).15 For example, 306-O12B is a bioreducible lipid with 12-carbon tails and amine 306 as the head group. The structural identity of amine head groups for the O-series ionizable cationic lipids can be found in Li et al. 2022.
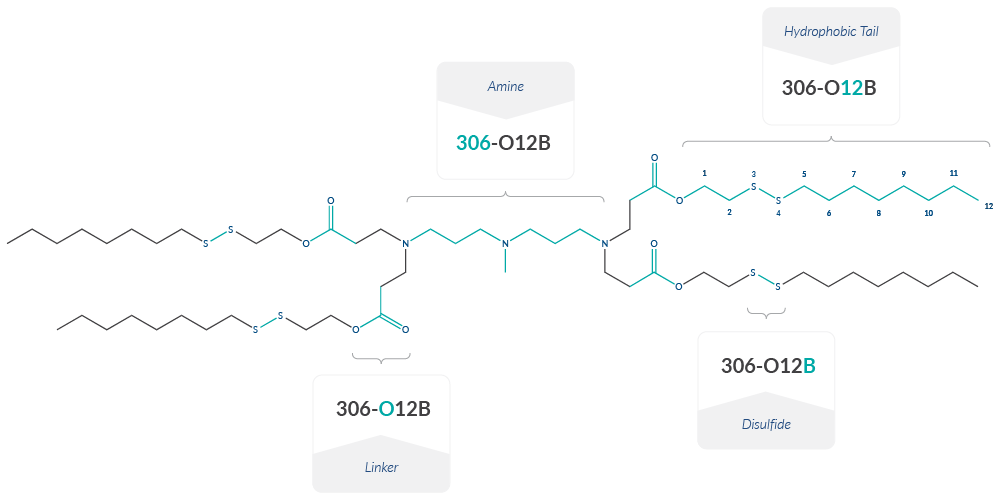
Figure 3. O-series lipid nomenclature using 306-O12B as an example. The structural identity of amine head groups for the O-series ionizable cationic lipids can be found in Li et al. 2022.
Intriguingly, both lipid classes can be tailored to target specific tissues, cells, and/or subcellular locations, including notoriously difficult targets. ssPalmA uses a vitamin A scaffold to target pDNA to the nucleus via cellular retinoic acid binding protein II (CRABP II).16 NT1-O14B harnesses a tryptamine-based head group to permit LNPs to cross the blood brain barrier, a major obstacle in the delivery of both small molecule inhibitors and RNA-based therapeutics to the brain.17 With this approach, LNPs formulated with NT1-O14B and 306-O12B were used to deliver antisense oligonucleotides (ASOs) against tau to the brain of mice, opening up an intriguing strategy for the treatment of Alzheimer's disease and other neurodegenerative conditions.
Other ionizable cationic lipids have been shown to target tissues in novel anticancer approaches. BAMPA-O16B can also be used to facilitate the passage of LNPs across the blood brain barrier.18 BAMPA-O16B-formulated LNPs loaded with siRNA targeting CD47 and programmed cell death protein ligand 1 (PD-L1) could cross the blood brain barrier, reduce tumor growth, and improve survival in a mouse model of glioblastoma multiforme, an aggressive brain cancer with poor prognosis. LNPs formulated with 113-O12B accumulate specifically in mouse lymph nodes.19 LNPs containing 113-O12B and encapsulating an mRNA cancer vaccine increase the number of tumoral CD8+ T cells and activated dendritic cells in a murine melanoma model. Hence, leveraging the diverse utility and tissue targeting ability of LNPs has the potential to lead the next generation of therapeutics and vaccines.
Rational and combinatorial design approaches
Both rational and combinatorial design approaches will continue to advance the development of ionizable cationic lipids. An ideal ionizable cationic lipid achieves the perfect combination of structural features and functional groups that balance safety and efficacy. Some basic structure-activity relationships can be made for ionizable cationic lipids, and strategically selected functional groups (e.g., vitamin A, tryptamine-based head groups) can boost their efficacy. Taken together, there is a place for a rational design approach in the synthesis of new ionizable cationic lipids. However, it is difficult to generalize most structure-activity relationships between ionizable cationic lipids. Each component influences the overall behavior of the lipid, and subtle changes in the structure of the lipid can impart large functional differences. Therefore, another approach is to synthesize large combinatorial libraries of chemically diverse lipids and then evaluate their activity with high-throughput screening approaches to identify the lead candidates.
| Contact our Chemical Synthesis Contract Services for the design and synthesis of custom LNP components. Our Chemistry team has decades of industry experience in performing complex and multi-step synthesis and can deliver high-purity LNP components. |
Cationic & Ionizable Cationic Lipids At-A-Glance
| Item No. | Item Name | Tertiary Amine | Ester | Disulfide |
| 37671 | 113-O12B | ✔ | ✔ | ✔ |
| 37549 | 306-O12B | ✔ | ✔ | ✔ |
| 37096 | 306-O12B-3 | ✔ | ✔ | ✔ |
| 37564 | 80-O16B | ✔ | ✔ | ✔ |
| 37095 | NT1-O14B | ✔ | ✔ | ✔ |
| 37377 | ssPalmM | ✔ | ✔ | ✔ |
| 37670 | ssPalmO-Phe | ✔ | ✔ | ✔ |
| 25726 | 1,2-Dioleoyl-3-dimethylammonium-propane (DODAP) | ✔ | ✔ | |
| 34366 | 93-O17O | ✔ | ✔ | |
| 34367 | 93-O17S | ✔ | ✔ | |
| 37903 | AA3-DLin | ✔ | ✔ | |
| 34337 | ALC-0315 | ✔ | ✔ | |
| 36935 | ATX-100 | ✔ | ✔ | |
| 37278 | CIN-16645 | ✔ | ✔ | |
| 37279 | CL4H6 | ✔ | ✔ | |
| 34364 | DLin-MC3-DMA | ✔ | ✔ | |
| 35051 | L-319 | ✔ | ✔ | |
| 35337 | Lipid 29 | ✔ | ✔ | |
| 34372 | Lipid 5 | ✔ | ✔ | |
| 37667 | Lipid A9 | ✔ | ✔ | |
| 37122 | Lipid C24 | ✔ | ✔ | |
| 33474 | SM-102 | ✔ | ✔ | |
| 37045 | TCL053 | ✔ | ✔ | |
| 15110 | 1,2-Dioleoyl-3-trimethylammoniumpropane (chloride) (DOTAP) | ✔ | ||
| 15109 | 1,2-Dioleyloxy-3-dimethylamino-propane (DODMA) | ✔ | ||
| 37276 | 9A1P9 | ✔ | ||
| 36699 | C12-200 | ✔ | ||
| 36700 | cKK-E12 | ✔ | ||
| 36701 | DLin-DMA | ✔ | ||
| 34363 | DLin-KC2-DMA | ✔ | ||
| 37652 | OF-02 | ✔ | ||
| 35786 | YSK05 | ✔ | ||
| 25926 | N-[1-(2,3-Dioleyloxy)propyl]-N,N,N-trimethylammonium (chloride) (DOTMA) |
You May Also Be Interested In
 A Guide to Lipid Nanoparticle Formulation |
 The Heads and Tails of Lipid-based Drug Delivery |
 Lipid Nanoparticle Development Services |
 Lipid Nanoparticle Research Tools |
References
1. Zhi, D., Bai, Y., Yang, J., et al. A review on cationic lipids with different linkers for gene delivery. Adv. Colloid Interface Sci. 253, 117-140 (2018).
2. Blanco, E., Shen, H., and Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotech. 33(9), 941-951 (2015).
3. Schlich, M., Palomba, R., Costabile, G., et al. Cytosolic delivery of nucleic acids: The case of ionizable lipid nanoparticles. Bioeng. Transl. Med. 6(2), e10213 (2021).
4. Zhang, Y., Dahal, U., Feng, Z.V., et al. Influence of surface ligand molecular structure on phospholipid membrane disruption by cationic nanoparticles. Langmuir 37(24), 7600-7610 (2021).
5. Miao, L., Zhang, Y., and Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 20(1), 41 (2021).
6. Bost, J.P., Barriga, H., Holme, M.N., et al. Delivery of oligonucleotide therapeutics: Chemical modifications, lipid nanoparticles, and extracellular vesicles. ACS Nano 15(9), 13993-14021 (2021).
7. Han, X., Zhang, H., Butowska, K., et al. An ionizable lipid toolbox for RNA delivery. Nat. Commun. 12(1), 7233 (2021).
8. Maier, M.A., Jayaraman, M., Matsuda, S., et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol. Ther. 21(8), 1570-1578 (2013).
9. Hou, X., Zaks, T., Langer, R., et al. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 6(12), 1078-1094 (2021).
10. Hassett, K.J., Benenato, K.E., Jacquinet, E., et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids 15, 1-11 (2019).
11. Sabnis, S., Kumarasinghe, E.S., Salerno, T., et al. A novel amino lipid series for mRNA delivery: Improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther. 26(6), 1509-1519 (2018).
12. Wang, M., Alberti, K., Varone, A., et al. Enhanced intracellular siRNA delivery using bioreducible lipid-like nanoparticles. Adv. Healthc. Mater. 3(9), 1398-1403 (2014).
13. Tanaka, H., Sakurai, Y., Anindita, J., et al. Development of lipid-like materials for RNA delivery based on intracellular environment-responsive membrane destabilization and spontaneous collapse. Adv. Drug Deliv. Rev. 154-155, 210-226 (2020).
14. Tanaka, H., Watanabe, A., Konishi, M., et al. The delivery of mRNA to colon inflammatory lesions by lipid-nano-particles containing environmentally-sensitive lipid-like materials with oleic acid scaffolds. Heliyon 4(12), e00959 (2018).
15. Li, Y., Ye, Z., Yang, H., et al. Tailoring combinatorial lipid nanoparticles for intracellular delivery of nucleic acids, proteins, and drugs. Acta Pharm. Sin. B 12(6),2624-2639 (2022).
16. Tanaka, H., Akita, H., Ishiba, R., et al. Neutral biodegradable lipid-envelope-type nanoparticle using vitamin A-Scaffold for nuclear targeting of plasmid DNA. Biomaterials35(5), 1755-1761 (2014).
17. Ma, F., Yang, L., Sun, Z., et al. Neurotransmitter-derived lipidoids (NT-lipidoids) for enhanced brain delivery through intravenous injection. Sci. Adv. 6(30),eabb4429 (2020).
18. Liu, S., Liu, J., Li, H., et al. An optimized ionizable cationic lipid for brain tumor-targeted siRNA delivery and glioblastoma immunotherapy. Biomaterials 287,121645 (2022).
19. Chen, J., Ye, Z., Huang, C., et al. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8+ T cell response. Proc. Natl. Acad. Sci.USA 119(34), e2207841119 (2022).










