Cayman Chemical Company,Inc.
入驻年限:4 年
- 联系人:
Kristina Whitfield
- 所在地区:
美国
- 业务范围:
试剂、抗体、细胞库 / 细胞培养、ELISA 试剂盒
- 经营模式:
生产厂商
推荐产品
公司新闻/正文
Solubility Factors When Choosing a Solvent
人阅读 发布时间:2021-09-24 15:32
Solubility Factors When Choosing a Solvent
Article from 2020-11-20
Solubility functions by a group of rules that determine how dissolvable a substance (solute) is in solvent and depends entirely on the physical and chemical properties of the solute and solvent. That is, solutes typically will dissolve best in solvents that have the most molecular similarities: polar solutes will dissolve better in polar solvents, and nonpolar solutes will dissolve better in nonpolar solvents. Additionally, solutes will be more soluble if the molecules in the solute are smaller than the ones in the solvent. This is because it is more difficult for solvent molecules to surround bigger molecules. Temperature also affects solubility in that solubility generally increases with heat except in the case of gases, which can become less soluble. Pressure is also a key factor in the solubility of a gas. Stirring or sonication are often necessary to increase the speed of dissolution, but they do not have an impact on the solubility of a substance. Because solubility is not affected by the rate of solution (how quickly a substance dissolves), rate should never be factored into decisions on the solubility of a substance.
| Cayman provides solubility data for most of our 17,000 biochemical compounds on each product page to help guide you in choosing a solvent. |
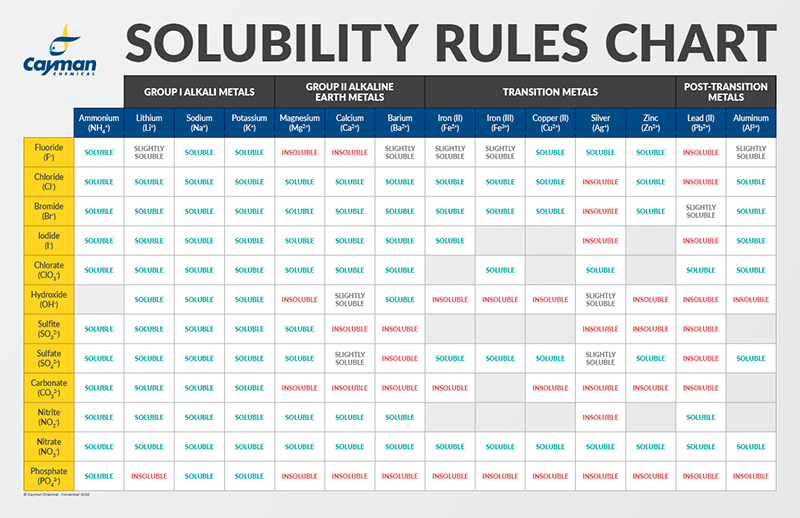
Solubility Guidelines*
*Pertains to substances in water at room temperature and standard pressure.
-
Salts containing Group I elements (Li+, Na+, K+, Cs+, Rb+) or ammonium ions (NH4+) are soluble.
-
Salts containing nitrates (NO3-), acetates (C2H3O2-), chlorates (ClO3-), and perchlorates (ClO4-) are generally soluble.
-
Binary compounds of halogens (Cl-, Br-, or I-) with metals are generally soluble. Exceptions to this include halide salts of fluoride (F-), silver (Ag+), lead (Pb2+), and mercury (Hg2+). Lead halides are, however, soluble in hot water. Most silver salts are insoluble except for AgNO3 and Ag(C2H3O2).
-
Most sulfate salts (SO42-) are soluble. Exceptions to this include CaSO4, BaSO4, Ag2SO4, HgSO4, and SrSO4, which are slightly soluble. PbSO4 is poorly soluble.
-
Most hydroxide salts (OH-) are insoluble. Exceptions include hydroxide salts of Group I elements, transition metals, aluminum, and ammonium. Hydroxide salts of Group II elements (Ca2+, Sr2+, and Ba2+) are only slightly soluble.
-
Most sulfides (S2-) of transition metals are highly insoluble, including CdS, FeS, ZnS, and Ag2S. Arsenic, antimony, bismuth, and lead sulfides are also insoluble. However, calcium (Ca2+), barium (Ba2+), strontium (Sr2+), magnesium (Mg2+), sodium (Na+), potassium (K+), and ammonium (NH4+) sulfides are soluble.
-
Carbonates (CO32-), oxalates (C2O42-), chromates (CrO42-), phosphates (PO42-), and fluorides (F-) are frequently insoluble. Although, compounds containing Group I elements or ammonium are soluble, except for lithium phosphate, which is poorly soluble.
With the chart below, the empirical formula of the compound can be used to determine solubility by cross referencing the cations (top row) with anions (first column). For less common compounds, you can consult a periodic table while using the solubility guidelines listed above.
Solvent Polarity
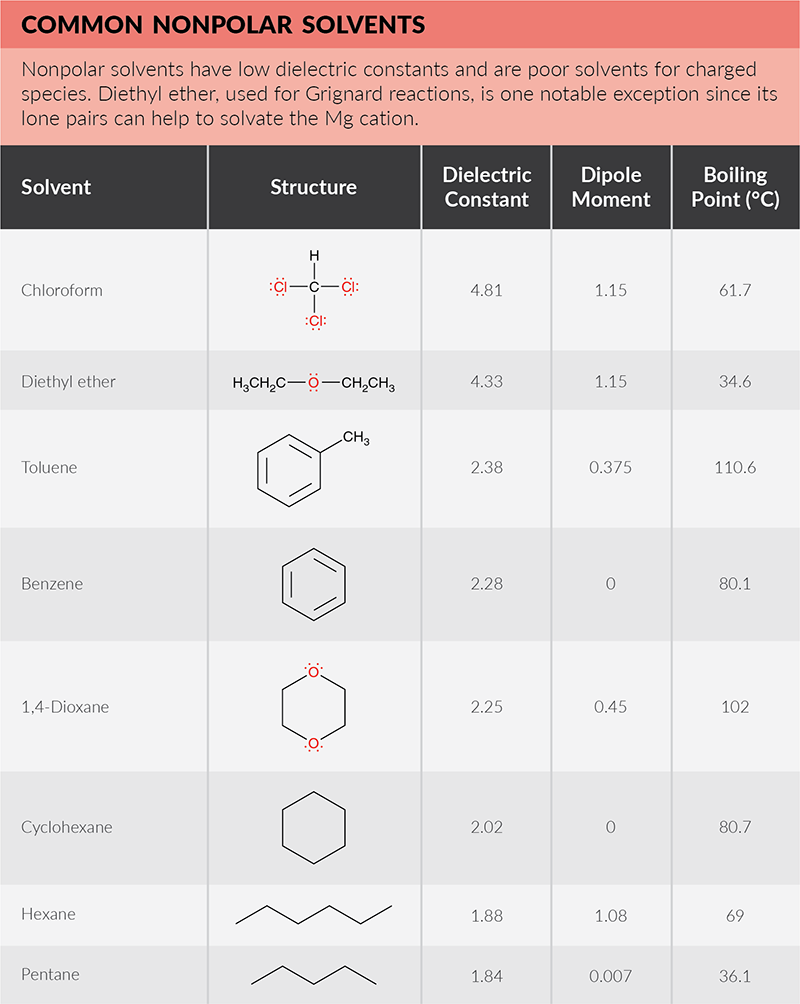
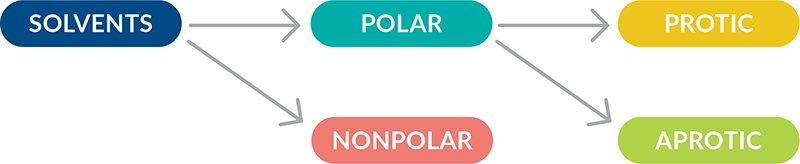
All solvents exist on a continuum of polarity. Solvents can be classified as polar, which contain bonds between atoms with very different electronegativities (such as O-H), and nonpolar, which contain bonds between atoms with similar electronegativities (such as C-H). As explained in detail below, polar solvents are classified as protic or aprotic. Polar protic solvents can form hydrogen bonds with water to dissolve in water and are best for dissolving polar reactants such as ions. Nonpolar solvents are not capable of strong hydrogen bonds and are better used for dissolving nonpolar reactants such as hydrocarbons.
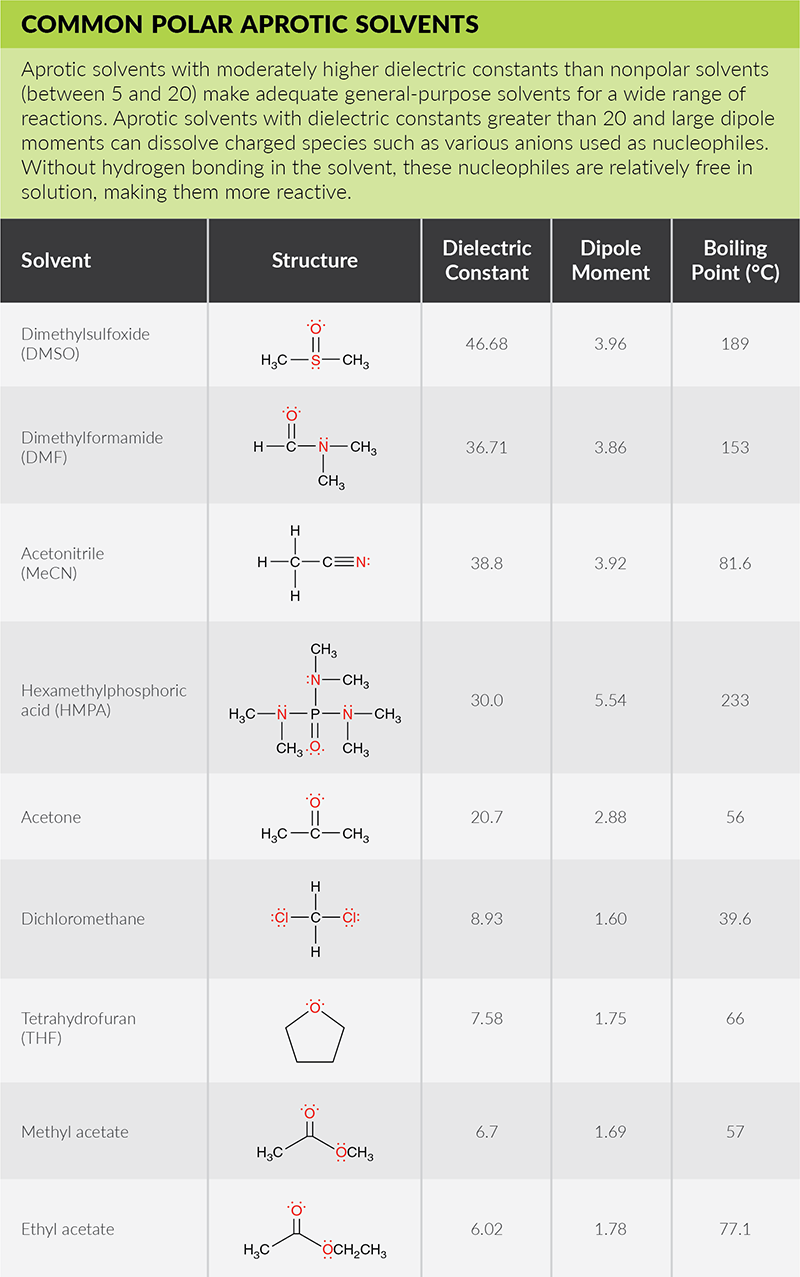
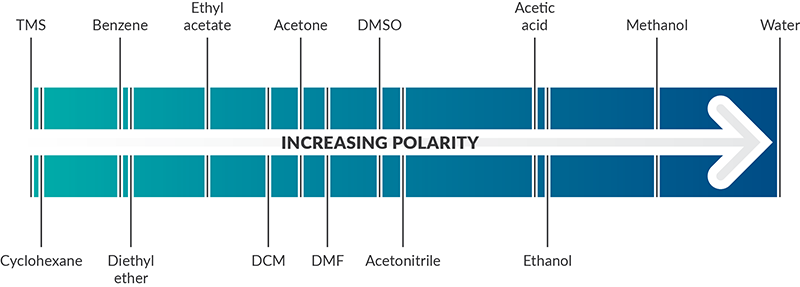
One way to measure polarity is through quantifying the dielectric constant (or relative permittivity)—the factor by which the electric field between two points of charges in the material is decreased relative to vacuum. The greater the dielectric constant, the greater the polarity. Solvents with dielectric constants less than five are considered nonpolar. A more direct way to measure polarity is by quantifying the dipole moment (or partial charges). Polar solvents have large dipole moments since they contain bonds with different electronegativities. Nonpolar solvents will lack partial charges and have little to no dipole moment. The below solvent polarity scale was derived from Reichardt’s dye to represent solvent polarity in correlation with chemical kinetics and equilibria (Sherwood, J. Bio-based solvents for organic synthesis, 2014).
Polar Solvents Are Either Protic or Aprotic
Polar protic solvents solvate cations and anions effectively. They are capable of hydrogen bonding because they contain at least one hydrogen atom connected directly to an electronegative atom (such as O-H or N-H bonds). In fact, the most common characteristic of a protic solvent is the presence of an OH group. Polar protic solvents can participate in reactions serving as a source of protons (acid), removing protons (base), or donating a lone pair of electrons as a nucleophile. As strong hydrogen bond donors, protic solvents are very effective at stabilizing ions in nucleophilic substitution reactions and favor reactions where ions are formed, like in SN1 reactions.
Polar aprotic solvents are not capable of hydrogen bonding, since they do not have hydrogen atoms connected directly to an electronegative atom. They only serve as a reaction medium and do not solvate nucleophiles. Aprotic polar solvents favor reactions where ions are reactants, like in SN2 reactions. SN2 reactions are significantly faster in polar aprotic solvents than in protic solvents.
Extenuating Conditions for Solubility
Even when using an appropriate solvent, the solubility of a substance can be affected by the solvent used for crystallization of the compound, residual solvent content, polymorphism, salt versus free form, degree of hydration, solvent temperature, and dissolved oxygen.
Solvents for Crystallization
Solids are purified through crystallization, which involves dissolving an impure solid in a solvent. For solids that are not sensitive to high temperatures, a minimal amount of hot solvent is added to the impure solid and, once dissolved, gradually allowed to cool to room temperature whereupon near pure crystals form. An ideal solvent for this process should allow the substance to dissolve at near boiling temperatures, while only enabling slight solubility at room temperature. At the same time, the dissolved impurities should be highly soluble in the solvent at both high and low solvent temperatures. The compound must precipitate out of solution at cooler temperatures to allow its separation from the solvent and the dissolved impurities by filtration. Sometimes a mixture of two or more solvents in a specific ratio is required to achieve this temperature-dependent solubility. For heat-sensitive compounds, a diffusion method can be used wherein the impure solid is dissolved in a minimum amount of solvent in which it is highly soluble. A solvent in which the compound is insoluble is slowly layered on top of this solution. Slow diffusion of the “insoluble” solvent into the “soluble” solvent will cause crystals to gradually grow at the interface between the two solvents. For diffusion to work, the impurities must be soluble in both solvents.
Common Solvents for Crystallization
Choosing a second solvent for a mixture usually takes trial and error, but there are some generally successful mixtures. For example, diethyl ether-methanol (or ethanol) is compatible for highly associated solids and many natural products. Diethyl ether-bezene works well for polar compounds and hydrocarbons.
| Solvent |
Compatible Compounds |
Second Solvent |
Notes |
| Water |
Salts, amides, some carboxylic acids |
Acetone, alcohols, dioxane, acetonitrile |
Precipitates dry slowly |
| Acetic acid |
Salts, amides, some carboxylic acids |
Water |
Difficult to remove; pungent odor |
| Acetonitrile |
Polar compounds |
Water, ethyl ether, benzene |
|
| Methanol |
General esters, nitro, and bromo compounds |
Water, ethyl ether, benzene |
|
| Ethanol |
General esters, nitro, and bromo compounds |
Water, hydrocarbons, ethyl acetate |
|
| Acetone |
General nitro and bromo compounds, osazones |
Water, hydrocarbons, ethyl ether |
Should be dried if not used with water |
| Methyl acetate |
General esters |
Water, ethyl ether |
|
| Ethyl acetate |
General esters |
Ethyl ether, hydrocarbons, benzene |
|
| Diethyl ether |
General, low-melting compounds |
Acetone, hydrocarbons |
May creep up the side of flask and deposit precipitate |
| Chloroform |
General, acid chlorides |
Ethanol, hydrocarbons |
Easily removed and dried |
| 1,4-Dioxane |
Amides |
Water, benzene, hydrocarbons |
May form solvates with some ethers |
| Toluene |
Aromatics, hydrocarbons |
Ethyl ether, ethyl acetate, hydrocarbons |
|
| Benzene |
Aromatics, hydrocarbons, molecular complexes |
Ethyl ether, ethyl acetate, hydrocarbons |
Cumulatively toxic |
| Pentane |
Hydrocarbons |
Ethanol, acetone, methyl acetate, ethyl acetate, diethyl ether, chloroform, 1,4-dioxane, toluene, benzene, hexane, cyclohexane |
|
| Hexane |
Hydrocarbons |
Ethanol, acetone, methyl acetate, ethyl acetate, diethyl ether, chloroform, 1,4-dioxane, toluene, benzene, pentane, cyclohexane |
|
| Cyclohexane |
Hydrocarbons |
Ethanol, acetone, methyl acetate, ethyl acetate, diethyl ether, chloroform, 1,4-dioxane, toluene, benzene, pentane, hexane |
|
Residual Solvent Content
Appropriate selection of a solvent for the synthesis of a compound is critical for the yield and/or determination of characteristics such as crystal form, purity, and solubility. However, by the completion of this synthetic process, it is possible that the solvent is not completely removed and will remain as an impurity. The United States Pharmacopoeia USP <467> Residual Solvents and ICH Q3C Guidelines for Residual Solvents provide allowable limits and testing criteria for residual solvents in pharmaceuticals based on the carcinogenicity, neurotoxicity, or teratogenicity of the solvent. The presence of these impurities may also have unintended effects in non-clinical use, so the reduction of their presence is critical. Residual solvents are typically identified by headspace gas chromatography (GC) along with identification and quantification using mass spectrometry (GC-MS). They can be minimized through various drying techniques.
Polymorphism
Polymorphism refers to the crystallization of the same compound into different crystalline forms. It is related to solvate formation, wherein molecules of solvent occur within the crystal structure. The removal or addition of solvents will change the structure of materials. Polymorphism occurs by different processing conditions such as pressure and temperature. Different polymorphs have different lattice energies that must be overcome to enter solution, so may have differences in dissolution. Note that the thermodynamically stable form of a compound is often the least soluble form.
Salt Versus Free Form
The use of a salt form of the free acid or free base (non-ionized form) facilitates solubility and rate of dissolution due to the buffering action of the salt. Typically, salts readily undergo crystallization. The ionic interactions in the salt crystal lattice increases hydrophilicity of a solid compound and can thus facilitate wettability. Moreover, the presence of dissolved counter ion shifts the pH towards higher solubility pH domains for a compound. A weak base formulated as a salt will have increased solubility and dissolution rate in a normal pH solution since upon dissolution the salt form will release protons to the medium decreasing the pH of the surrounding environment. Conversely, the dissolution of weak acids in salt form will increase the pH of the surrounding environment in a highly acidic solution. Free acids are likely to be more soluble in a nonpolar solvent. Be aware that enhancing the dissolution rate can result in a level of supersaturation that may lead to precipitation of the free form out of solution.
Degree of Hydration
Water is one of the few liquids that readily dissolves many ionic solids. Few other molecules are both small enough and polar to cluster around positive and negative ions in solution. When ions are dissolved in water, they attract and hold several water dipoles around them. The negative (oxygen) side of a dipolar water molecule attracts and is attracted by any positive ion in solution. Because of this ion-dipole force, water molecules cluster around positive ions. Similarly, the positive (hydrogen) ends of water molecules are attracted to negative ions. The process by which either a positive or a negative ion attracts water molecules to its immediate vicinity is termed hydration. Hydration of cations and anions in aqueous solution prevents them from attracting each other into a crystal lattice and precipitating. The degree of hydration depends upon the size of the ion. As size increases hydration energy decreases. As water molecules are attracted to ions, there is a net lowering of the potential energy of the compound particles and heat energy is given off. The significant hydration energy of these counter ions typically contributes to the overall net gain in free energy levels during the dissolution process.
Solvent Temperature
Although some solutes exhibit solubility that is independent of temperature, the solubility of many substances increases with increasing solvent temperature. Increased kinetic energy or vibration generated by higher temperatures allows the solvent molecules to more effectively break apart the solute molecules that are held together by intermolecular attractions. Additionally, the increased kinetic energy of the solute molecules causes them to dissolve more readily because they become destabilized. Some compounds can also demonstrate inverse solubility, becoming less soluble in water as temperature increases. This occurs when the dissolution of a compound is exothermic. Extra heat causes the equilibrium for an exothermic process to shift towards the reactants.
Dissolved Oxygen
Dissolved oxygen refers to the level of free oxygen not bonded to any other element present in water or other solvents. Its presence may expose sensitive compounds to oxidation, which could lead to corrosion, undesired pH levels, or unwanted byproducts. Dissolved oxygen is often removed by degassing systems for analytical or high-purity processes.
Additional Considerations
Even when the most careful considerations outlined here are given to select an appropriate solvent, compounds in solution may become unstable. It is best practice to make fresh solutions for your experiments or store pre-made solutions at -80°C.